BerkiGEM2007Present6
From 2007.igem.org
(→Summer Work) |
(→Novelty and Non Obvious) |
||
| Line 17: | Line 17: | ||
==Novelty and Non Obvious== | ==Novelty and Non Obvious== | ||
| + | There are existing patents relating to different types of oxygen therapeutics. These patents include PFC compunds’ as oxygen therapeutics and methods to maximize the production yields of hemoglobin using ''Escherichia coli'' expression systems. The application of Bacto Blood’s ''E. coli'' must be both novel and non-obvious over the prior art. The novelty and nonobviousness of Bacto Blood relative to other oxygen therapeutics lies in the expression of hemoglobin in an ''E. coli'' system that is genetically engineered to be safe ''in vivo'' human therapy. Bacto Blood is novel because the team created biological parts that can be used to suppress the normal replication cycle of ''E. coli'' so that it does not cause sepsis in the human body. The “aseptic” bacteria were then combined with other biological parts created by different team members. The parts and the different devices generated by the combinations of parts such as, (an oxygen carrier, a controller, a self-destruct mechanism, and a freeze drying component) were constructed and inserted into the “aseptic” ''E. coli''. | ||
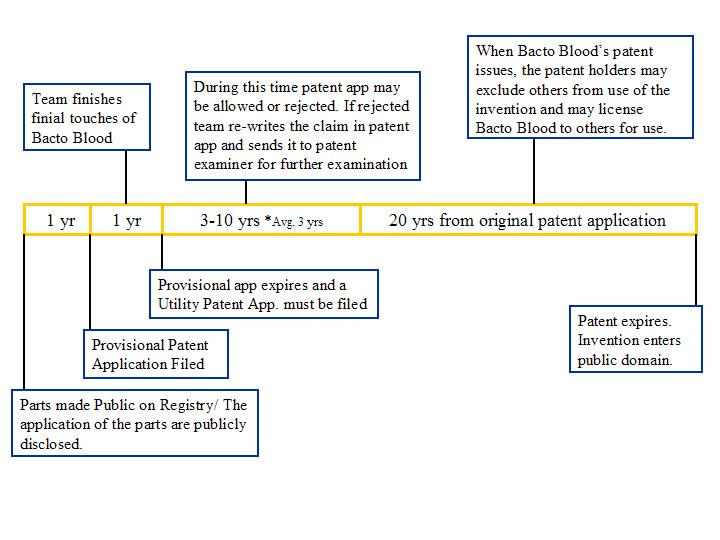
==Patent application timeline== | ==Patent application timeline== | ||
Revision as of 03:32, 25 October 2007
<< Back to UC Berkeley iGEM 2007
<< Back to Setup
Human Practices
Human Practices examines the way synthetic biology might inform human security, health, and welfare through the new objects that synthetic biology brings into the world. It also observes the ways in which economic, political, and cultural forces may shape the development of synthetic biology.
Summer Work
I focused my investigation on the patentability of Bacto Blood where its parts are in an open source forum given that there are existing patents on oxygen-based therapeutics. If it were possible to patent Bacto Blood, how could my team obtain patent protection for their product? Bacto Blood is patentable despite having its parts listed an open source forum. However, patentability of Bacto Blood may be complex. Two questions that read on Bacto Blood’s patentability are:
1. What aspects of Bacto Blood are patentable, the individual part(s), the part(s) as put together into the whole, or the applications made possible by the part(s)?
2. What starts the timeline for patenting Bacto Blood: when the part is put on the registry or when the applications of the part is made public?
Novelty and Non Obvious
There are existing patents relating to different types of oxygen therapeutics. These patents include PFC compunds’ as oxygen therapeutics and methods to maximize the production yields of hemoglobin using Escherichia coli expression systems. The application of Bacto Blood’s E. coli must be both novel and non-obvious over the prior art. The novelty and nonobviousness of Bacto Blood relative to other oxygen therapeutics lies in the expression of hemoglobin in an E. coli system that is genetically engineered to be safe in vivo human therapy. Bacto Blood is novel because the team created biological parts that can be used to suppress the normal replication cycle of E. coli so that it does not cause sepsis in the human body. The “aseptic” bacteria were then combined with other biological parts created by different team members. The parts and the different devices generated by the combinations of parts such as, (an oxygen carrier, a controller, a self-destruct mechanism, and a freeze drying component) were constructed and inserted into the “aseptic” E. coli.