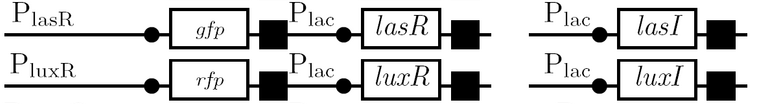Construction and Testing
From 2007.igem.org
(Difference between revisions)
(→Testing functionality of the various promoters) |
(→Testing functionality of the various promoters) |
||
| Line 45: | Line 45: | ||
<td>[[Image:UW_testing_constructs2A1A2.png|400px]]</td> | <td>[[Image:UW_testing_constructs2A1A2.png|400px]]</td> | ||
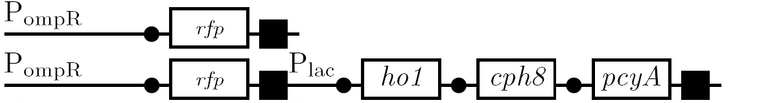
<td><b>The Omp promoter</b><br> | <td><b>The Omp promoter</b><br> | ||
| - | * | + | *positively-controlled promoter activated by endogenous OmpR protein (sensitive to salt concentrations via EnvZ protein) |
*test for activity using high salt levels in EnvZ+ strain | *test for activity using high salt levels in EnvZ+ strain | ||
*test for activity using the photo-active red system in EnvZ- strain </td> | *test for activity using the photo-active red system in EnvZ- strain </td> | ||
| Line 58: | Line 58: | ||
<td>[[Image:UW_testing_constructs2CD.png|400px]]</td> | <td>[[Image:UW_testing_constructs2CD.png|400px]]</td> | ||
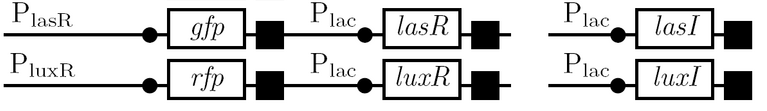
<td><b>The Plas and Plux quorum-sensing promoters </b><br> | <td><b>The Plas and Plux quorum-sensing promoters </b><br> | ||
| - | *as | + | *test for levels of expression in the unenhanced versus enhanced state |
| + | *lasR/luxR must be expressed in same strain as Plas/Plux since these genes encode transcription activators regulated by the products of lasI/luxI; lasI/luxI can be expressed in a different strain since their respective AHL molecules are diffusable through cell membranes | ||
| + | *the two strains are grown in a single culture tube for this test</td> | ||
</tr> | </tr> | ||
<tr valign="middle" style="border-top: 1px solid black; border-bottom: 1px solid black;"> | <tr valign="middle" style="border-top: 1px solid black; border-bottom: 1px solid black;"> | ||
<td>[[Image:UW_testing_constructs2E.png|400px]]</td> | <td>[[Image:UW_testing_constructs2E.png|400px]]</td> | ||
<td><b>The PlasOcI promoter-operator fusion </b><br> | <td><b>The PlasOcI promoter-operator fusion </b><br> | ||
| - | *negatively- and positively-controlled promoter in the final half adder design, repressed by cI and enhanced by LasR | + | *both negatively- and positively-controlled promoter in the final half adder design, repressed by cI and enhanced by LasR |
| - | * | + | *test for activity in the repressed versus enhanced state |
| - | *test in addition to above construct since operator fusion may affect promoter activity </td> | + | *test in addition to above construct since cI operator fusion may affect <i>las</i> promoter activity </td> |
</tr> | </tr> | ||
</table> | </table> | ||
Revision as of 13:58, 26 October 2007
Contents |
Construction Tree
The diagram below outlines our parallel construction plan, from individual registry parts to the final half-adder. Because many people were working on the project at once, it is designed to keep everyone organised.
Diagram details:
- Expected fragment sizes are shown at every node so gel results can be analyzed quickly.
- Vector resistances for each part/construct are shown, with shades of blue representing ampicillin and shades of red representing kanamycin.
- Parts and developing constructs all have abbreviated names for ease of labeling. To make the subtle distinction between a coding sequence and a coding sequence with a ribosome binding site, we use a ' on the computer and an underline when writing. A double ' or double underline indicates a coding sequence with a ribosome binding site and a transcriptional terminator.
- Arrows show the direction of DNA cloning and specify which restriction enzymes to use for cutting. Hollow arrows are present when two parts are ligated from vectors of the same resistance, requiring additional methods such as the use of alkaline phosphatase, gel extraction, or a three-way ligation into a vector of difference resistance.
- The numbers 1-2-3-3.5-4 show the major protocols that need to be carried at each step in the assembly cycle.
Testing Constructs
We use the fluorescent proteins GFP and RFP as reporter genes to test the functionality of some of the components and constructs of our half-adder design. Where possible, we make use of the lac inducible promoter to control expression of the enhancing or repressing elements.
Comparing basal and induced expression levels for the quorum sensing promoters
Testing functionality of the various promoters
Home