Alberta
From 2007.igem.org
(→'''Project Timeline''') |
(→'''Project Timeline''') |
||
| Line 38: | Line 38: | ||
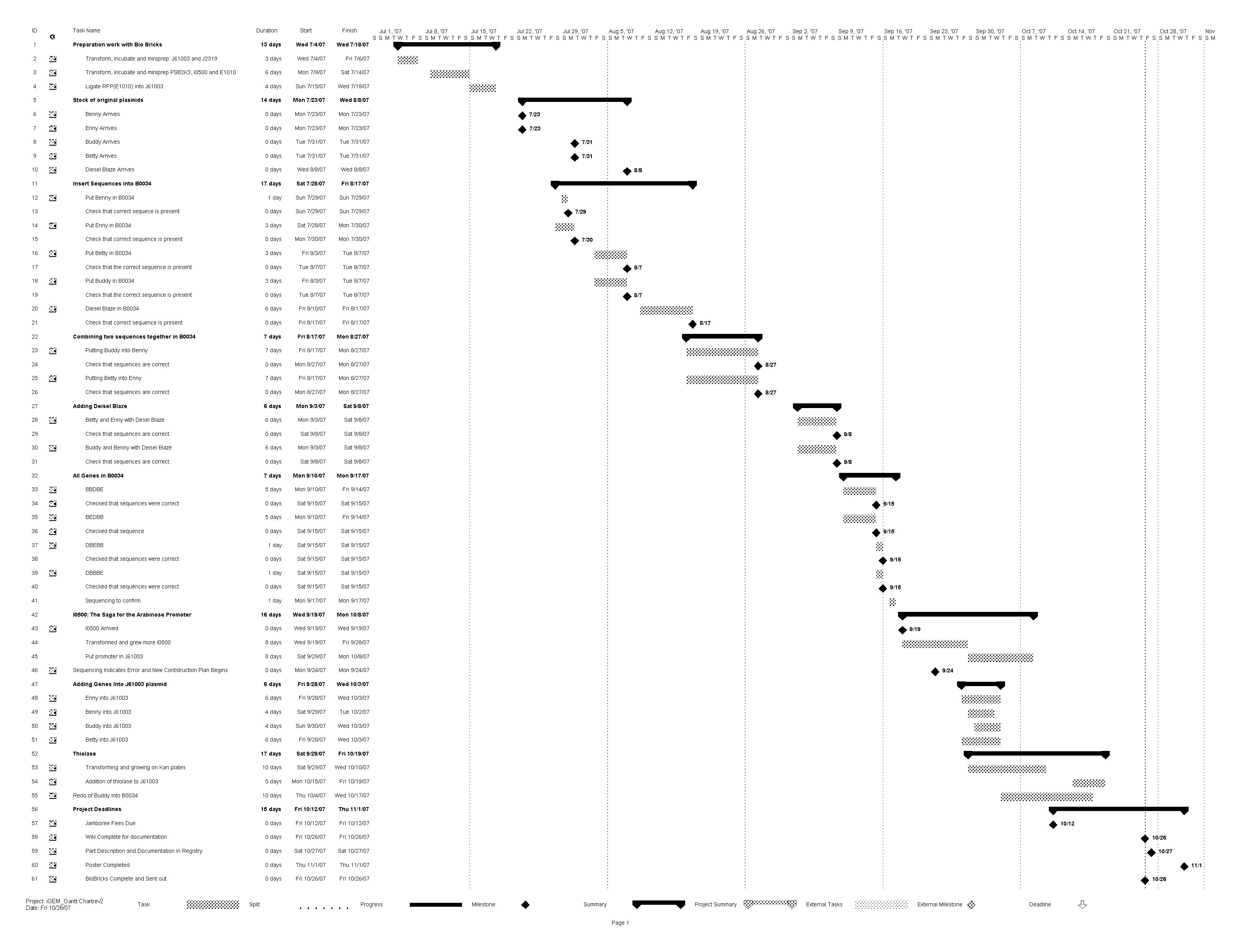
== '''Project Timeline''' == | == '''Project Timeline''' == | ||
| - | [[image: Alberta_gantt.jpeg|thumb| | + | [[image: Alberta_gantt.jpeg|thumb|600px|Center|Project Gantt Chart (click to Enlarge]] |
| + | |||
| + | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
== '''Lab Book and Calendar''' == | == '''Lab Book and Calendar''' == | ||
Revision as of 15:33, 26 October 2007

The Offical Wiki of the 2007 University of Alberta iGEM Team
Contents |
Background: Biofuels
With growing concerns of global greenhouse gasses, the global energy market is in a continual push for the development of renewable energy sources. Specifically, two fuels have dominated the media: biodiesel and ethanol. However, both of these fuels have shortcomings in terms of being a viable fuel source.
Biodiesel is a fuel produced from the vegetable oils of crops that can be used in an engine system very similar to a traditional diesel engine. However, vegetable oils in crops only make up a small portion of the biomass of the plants, ultimately producing low yields of fuel per acre of crops. As such, it is more economically sound to use these resources for food production.
There has been huge attention to the use of ethanol in the typical auto cycle engine. In many places it is already being blended with gasoline to create a hybrid fuel source. However, running an engine on pure ethanol is beset by several major obstacles. Firstly, ethanol is miscible with water at any concentration, which creates long term storage corrosion issues. Secondly, ethanol engines must be designed to expect water vapor unlike their gasoline counterparts. Ethanol also has significantly different thermodynamic properties than gasoline such as a lower energy density and different vapor properties, which would reduce the economical advantage of using ethanol as a primary fuels source.
We propose using a different fuel source to eventually replace gasoline, butanol. Butanol is a superior to ethanol as a replacement for petroleum gasoline. With a low vapor pressure, high energy density, and a gasoline-like octane rating, it can be blended into existing gasoline at much higher proportions than ethanol without compromising performance, mileage, cold starting, or volatile organic pollution standards. Blending butanol with gasoline also prevents modifications of the fuel-air ratio and modifications to the fuel system. Butanol also is immiscible with water at concentrations higher than 7%, alleviating storage concerns.
More information on the summary of biofuels viability and the inspiration to our project can be found here.
The Project: Plan B
We propose the use of butanol as the leading biofuel for use in internal combustion engines. Specifically, we intend to genetically engineer Escherichia coli bacteria to convert biomasses into butanol for use as an energy source. This will be accomplished by introducing the genes responsible for butanol production from Clostridium acetobutylicum into E. coli. Furthermore, we hope to increase E. coli's tolerance to solvents such as butanol.
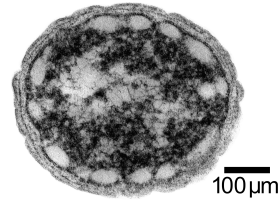
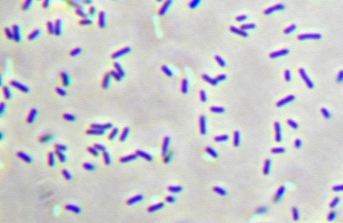
Concurrently, we are investigating the use of a photoautotrophic bacterium, Chlorobium tepidum, that we will also introduce butanol producing genes to. Chlorobium tepidum is a green sulfur bacterium that is strictly anaerobic and uses sulfur compounds as terminal electron donors. Chlorobium tepidum is a moderately thermophillic bacterium, growing at 40 degrees celcius (104 degrees fahrenheit), and requires low light conditions for optimal growth. These bacteria grow well in a defined medium, utilizing the reverse/reductive tricarboxylic acid (TCA) cycle to build up carbohydrates from carbon dioxide. For a more comprehensive overview of Chlorobium tepidum go to http://www.bmb.psu.edu/faculty/bryant/lab/chlorobiumtepidum/index.html
Figure 1 and 2 Images from http://www.bmb.psu.edu/faculty/bryant/lab/chlorobiumtepidum/index.html
The advantage of manipulating this organism for butanol production is a matter of energy input and output. Rather than having to utilize vast amounts of food stocks, such as grains or sugars, a photoautotroph will fix carbon dioxide into the complex carbohydrates required for butanol production. Theoretically, if the only fuel on our planet was butanol, and it was produced in this manner, there would be little net carbon dioxide produced.
Project Timeline
Lab Book and Calendar
Butanerd Event Calendar can be found below:
[http://www.ualberta.ca/~mjl3/UofAIgemProtocols.pdf The Lab Protocols]
Discussion Board
[http://butanerds.myfreeforum.org The Butanerd Online Forum] is up and running. Feel free to post and check for posts here. Make sure you register!
The Team

Our team has a rich background in biology, biochemistry and engineering. To compliment our diversity we also have advisors who have a wealth of knowledge in research and applications of genetic engineering. For more information about the group, check out the University of Alberta's iGEM Team Members Page.
Our University of Alberta Student Group Constitution can be found here
Edmonton
Welcome to Edmonton!

For more on the city of Edmonton click [http://www.ualberta.ca/~mjl3/About.html here].
Computational Modeling
Initially our plan to model the efficiency of the butanol production hinged on developing a complete model for the entire glucose pathway in an E. coli cell using Michaelis-Menten kinetics. After further review, this proved to be beyond the scope of our project's timeline due both to the large number of species and reactions present in the system (as well as their non-linear behavior) and the relative difficulty in finding experimental kinetic information for some of the reactions in our pathway.
Therefore, to obtain a rough "first-order" approximation for the behavior of the pathway, we considered two factors:
1) Stoichiometric factors (both redox and carbon) and
2) Compared the relative production rates of the various end products of the glucose pathway in E. coli as found in previous experimentation to the production rates of butanol in C. acetobutylicum.
From these factors we can obtain an indicator of how likely butanol will be produced.
In order to further develop the system, we hope a full metabolic stochiometric model can be eventually realized for this project. This would allow optimization of the system in order to maximize the flux through the butanol pathway.
Sponsors
We would like to thank all of our sponsers for their gracious support and our advisors for invaluble advise.
Major Sponsors

|
Sponsors

|
Advice
Andrew Hessel - Alberta Ingenuity Mentor,
Dr. Jonathan Dennis - University of Alberta,
Dr. Perrin Beatty - University of Alberta,
Dr. David Bressler - University of Alberta,
Dr. Charles Lucy - University of Alberta,
Dr. Gregory Kiema - University of Alberta,
Dr. Mark S. Peppler - University of Alberta,
Dr. James Harynuk - University of Alberta,
Dr. Federick West-University of Alberta,
Dr. Todd Lowary-University of Alberta,
Desiree Schell - University of Alberta CJSR
Dr. Jeff Fuller - University of Alberta/Capital Health
Dr. Julia Foght - University of Alberta
Dr. David Checkel - University of Alberta
Adrian Audiet - University of Alberta
Dr. Koch - University of Alberta
Dr. Donald Bryant - Penn State,
Dr. Gaozhong Shen - Penn State,
Amaya Garcia - Penn State,
A list of experimental supplies we need can be found here
External Links
[http://www.albertaingenuity.ca/ Alberta Ingenuity Fund]
[http://www.ualberta.ca The University of Alberta Hompage]
[http://www.mece.ualberta.ca/~ckoch/ Dr. Koch's Engine Lab]
[http://www.systems-biology.org/cd Cell Designer Homepage]
[http://www.biomodels.org Biomodels Home Page]