Chiba/Engeneering Flagella
From 2007.igem.org
(→Results) |
|||
| Line 8: | Line 8: | ||
| - | ==Our Aim== | + | ==Stickey Tags== |
| + | ===Our Aim=== | ||
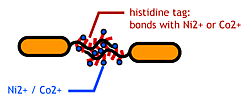
[[Image:Chiba_stickbacteria.png|frame|'''Fig1.'''Bacteria Linker]] | [[Image:Chiba_stickbacteria.png|frame|'''Fig1.'''Bacteria Linker]] | ||
To make stickey hands on ''E.coli'', we focused on their flagella that are located outside the cells. We used the following mechanisms: | To make stickey hands on ''E.coli'', we focused on their flagella that are located outside the cells. We used the following mechanisms: | ||
| Line 15: | Line 16: | ||
We combined these two and made a His-tagged flagella in the hope to stick them together via metal ions. | We combined these two and made a His-tagged flagella in the hope to stick them together via metal ions. | ||
| - | ==About flagella== | + | ===About flagella=== |
''E.Coli'' have 5-10 flagella. | ''E.Coli'' have 5-10 flagella. | ||
The flagella is used for swimming and for chemotaxis; the bacteria run when they find attractant, tumble when there is a repellent. | The flagella is used for swimming and for chemotaxis; the bacteria run when they find attractant, tumble when there is a repellent. | ||
| Line 30: | Line 31: | ||
D1とD2はformation of the functional flagellar formation(ffff)の為に必要であるが,D3ドメインは必要でなく可変である(図:蛋白質構造&鞭毛断面図) | D1とD2はformation of the functional flagellar formation(ffff)の為に必要であるが,D3ドメインは必要でなく可変である(図:蛋白質構造&鞭毛断面図) | ||
| - | ==="Variable" FliC D3 domain=== | + | ===="Variable" FliC D3 domain==== |
It is possible to display proteins(up to 49.4kDa)on the cell surface of E.Coli using flagellin fusion protein.英語変. | It is possible to display proteins(up to 49.4kDa)on the cell surface of E.Coli using flagellin fusion protein.英語変. | ||
可変なD3ドメインに他の<__aaまでの>アミノ酸を挿入しても鞭毛が合成される[2].詳細も書く. | 可変なD3ドメインに他の<__aaまでの>アミノ酸を挿入しても鞭毛が合成される[2].詳細も書く. | ||
| - | ===References=== | + | ====References==== |
#Kuwajima, G. ''et al''.: Nature Biotechnology, 6, 1080-1083 (1988). | #Kuwajima, G. ''et al''.: Nature Biotechnology, 6, 1080-1083 (1988). | ||
#Tanskanen, J. ''et. al''.: Appl. and Env. Microbiol., 4152-4156 (2000) | #Tanskanen, J. ''et. al''.: Appl. and Env. Microbiol., 4152-4156 (2000) | ||
| - | ==About Histidine Tag== | + | ===About Histidine Tag=== |
See [http://en.wikipedia.org/wiki/His-tag wikipedia article]. | See [http://en.wikipedia.org/wiki/His-tag wikipedia article]. | ||
Revision as of 19:01, 26 October 2007
|
Introduction | Project Design ( 1.Sticky Hands | 2.Communication | 3.Size Control ) | Making Marimos | Our Goal || Team Members | メンバ連絡簿 |
Stickey Tags
Our Aim
To make stickey hands on E.coli, we focused on their flagella that are located outside the cells. We used the following mechanisms:
- Display sticky peptides in flagellar filament.
- The imidazole group in histidines make a complex with metal ions.
We combined these two and made a His-tagged flagella in the hope to stick them together via metal ions.
About flagella
E.Coli have 5-10 flagella. The flagella is used for swimming and for chemotaxis; the bacteria run when they find attractant, tumble when there is a repellent.
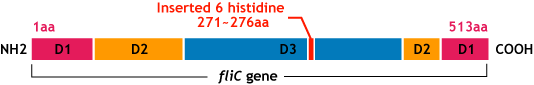
E.coli flagella consist of three parts: a basal body, a hook, and a filament. The filament of E.Coli is a highly rigid, helical, and cylindrical structure which is 10-15μm long and 23nm thick in diameter. It is built from ~20000 subunits of a ~55kDa single protein, FliC. FliC has three domains, D1,D2,D3. Although D1 and D2 are needed for the formation of the functional flagellar filament, D3 does not take part in the formation of flagella. (Ref)
大腸菌は5~10本/細胞の鞭毛を持つ.鞭毛を回転/逆回転させることにより環境の良い場所へとtaxisする. 鞭毛は長さ約10~15μm,半径約23nmの空孔のチューブ状である.(図:菌全体) 鞭毛のフィラメント部はFliCという蛋白質が規則正しく配列した多量体である.(図:鞭毛アップ) FliCはD0,D1,D2,D3ドメインを持つ.(種類によってはD1,D2,D3の3つのドメインを持つ) D1とD2はformation of the functional flagellar formation(ffff)の為に必要であるが,D3ドメインは必要でなく可変である(図:蛋白質構造&鞭毛断面図)
"Variable" FliC D3 domain
It is possible to display proteins(up to 49.4kDa)on the cell surface of E.Coli using flagellin fusion protein.英語変.
可変なD3ドメインに他の<__aaまでの>アミノ酸を挿入しても鞭毛が合成される[2].詳細も書く.
References
- Kuwajima, G. et al.: Nature Biotechnology, 6, 1080-1083 (1988).
- Tanskanen, J. et. al.: Appl. and Env. Microbiol., 4152-4156 (2000)
About Histidine Tag
See [http://en.wikipedia.org/wiki/His-tag wikipedia article].
Experiments
Making FliC-his gene
- FliC遺伝子のD3領域に、Histidineループをコードする遺伝子を挿入し、ヒスチジンタグとして発現させる。
We inserted the gene of six histidine loop peptide (“His-Tag”).
![]() Sequence Confirmed
Sequence Confirmed
![]() Swarm Confirmed
Swarm Confirmed
![]() Flagella strained with anti-flagella antibody
Flagella strained with anti-flagella antibody
Display Check: Beads Adsorption
Purpose
Confirm that the his-tag is displaied on the flagella surface of E.Coli.
Samples
- JW1908(⊿fliC株)
- pUC19-fliC-his
- plasmidなし
- GI826(⊿fliC⊿motB株)
- pUC19-fliC-his
- plasmidなし
Procedure
- pUC19-FliC-His was transformed to JW1908(fliC) and GI826(fliC motB)
- 培養液をビーズと懸濁させ、大腸菌をBeadsに吸着する。
- 吸着した大腸菌をbufferで溶出し、incubate。
Results
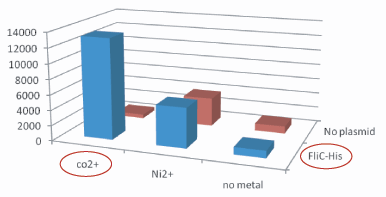
- strainの比較(データ?)
|
Strain |
Co2+ |
No metal |
|
JW1908 |
吸着していない |
吸着していない |
|
GI826 |
吸着していた |
吸着していない |
(Negative Control 1:Noplasmid,Negative Control 2:Co2+あるいはNi2+の有り・無し) →吸着していなかった (Negative Control 1:Noplasmid,Negative Control 2:Co2+あるいはNi2+の有り・無し) →吸着していた。
Plasmidあり、かつCo2+つきビーズ、のコロニー数は、その他のものよりはるかに多い。30倍以上。 Histidine Tagの存在によって大腸菌がビーズに吸着吸着していることがわかる。 The number of colony dramatically decreased with out Co2+ or FliC-His plasmid.
- 考察も書いたら? なぜコバルトがニッケルより良いのか?→一般的にニッケルよりコバルトの方が結合力が強いことが知られてて,FilChisに関してもそれは変わらないみたいな・・・by tashiro