ETHZ/Biology
From 2007.igem.org
(→.:: System parts ::.) |
m (Fixed headings, removed old Stefan's text.) |
||
| Line 9: | Line 9: | ||
<p>In this page, you can find an analysis of the function of our system and its relation to epigenetics, its biological design and a list of the parts that it consists of. Are you interested in constructing educatETH <i>E.coli</i> in your lab? Then under [https://2007.igem.org/ETHZ/Biology/Lab In the Lab], you can find the ingredients and equipment we used, the electronic version of our lab notebook and a presentation of all the difficulties that we encountered. If you are also interested on how educatETH ''E.coli'' was simulated outside the lab, please visit the [[ETHZ/Engineering | Engineering Perspective]]. </p><br> | <p>In this page, you can find an analysis of the function of our system and its relation to epigenetics, its biological design and a list of the parts that it consists of. Are you interested in constructing educatETH <i>E.coli</i> in your lab? Then under [https://2007.igem.org/ETHZ/Biology/Lab In the Lab], you can find the ingredients and equipment we used, the electronic version of our lab notebook and a presentation of all the difficulties that we encountered. If you are also interested on how educatETH ''E.coli'' was simulated outside the lab, please visit the [[ETHZ/Engineering | Engineering Perspective]]. </p><br> | ||
__TOC__ | __TOC__ | ||
| - | == | + | == Introduction == |
<p> educatETH <i>E.coli</i> is a system which can distinguish between aTc and IPTG based on a previous training phase conducted with the same chemicals and the help of AHL. It composes of three subsystems: the subsystem of constitutively produced proteins, the learning subsystem and the reporting subsystem. The constitutively produced proteins (lacI, TetR and LuxR) control the learning subsystem. At the core of the latter there exists a modified version of the toggle switch found in [1] with two operator sites, so that it only changes its state when both one of the two chemicals (aTc/IPTG) and AHL are present. As AHL is only present during the training phase, the toggle maintains its state during testing, and thus can “memorize”. In the reporting subsystem, four reporters allow supervision of both the chemical the system was trained with and of if the system recognizes the chemical it is being exposed to in the testing phase as one it has been trained with or not.</p><br> | <p> educatETH <i>E.coli</i> is a system which can distinguish between aTc and IPTG based on a previous training phase conducted with the same chemicals and the help of AHL. It composes of three subsystems: the subsystem of constitutively produced proteins, the learning subsystem and the reporting subsystem. The constitutively produced proteins (lacI, TetR and LuxR) control the learning subsystem. At the core of the latter there exists a modified version of the toggle switch found in [1] with two operator sites, so that it only changes its state when both one of the two chemicals (aTc/IPTG) and AHL are present. As AHL is only present during the training phase, the toggle maintains its state during testing, and thus can “memorize”. In the reporting subsystem, four reporters allow supervision of both the chemical the system was trained with and of if the system recognizes the chemical it is being exposed to in the testing phase as one it has been trained with or not.</p><br> | ||
| - | == | + | == The complete system == |
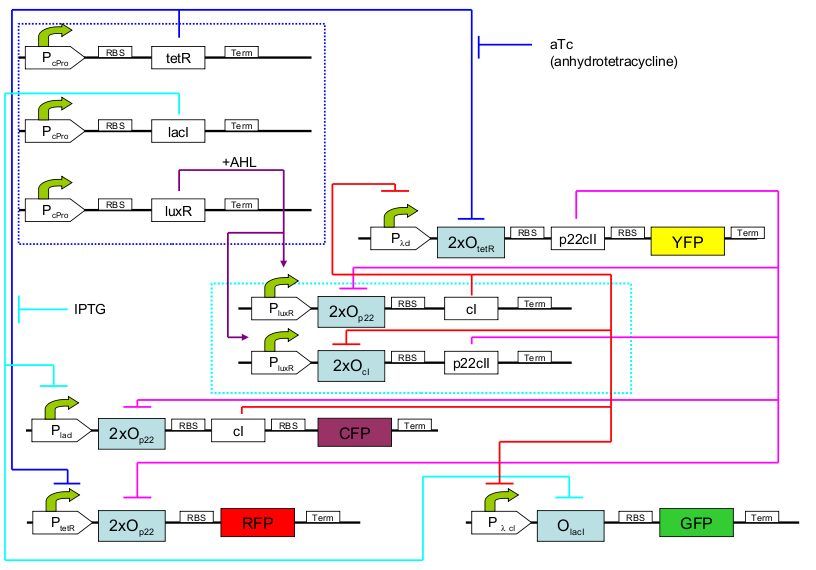
| - | <p>The biological design of | + | <p>The biological design of EducatETH <i>E.coli</i> is presented on [[Image:new_learning_system3.png|thumb|left|300px|'''Fig. 1: '''educatETH ''E.coli'' System]] . In the following, we will clarify the function of all depicted components. (Are you interested in how the complex system of Fig. 1 was modelled? Then visit the [[ETHZ/Engineering | Engineering Perspective]]!)</p><br> |
| - | === | + | === Constitutive subsystem === |
<p>The constitutively produced proteins of the system are LacI, TetR and LuxR. The LuxR part has a special function: when AHL is present, it forms a LuxR-AHL complex which acts on the learning subsystem (more on this later). At the moment, we will consider that AHL is absent and therefore LuxR cannot act on any subsystems. The TetR and lacI parts behave similarly: more specifically, the tetR protein in the absence of aTc inhibits the production of p22cII and LacI in the absence of IPTG inhibits the production of cI. When aTc is present, however, the p22cII production is no longer inhibited (and thus aTc is produced). Respectively, cI is produced when IPTG is present.</p> | <p>The constitutively produced proteins of the system are LacI, TetR and LuxR. The LuxR part has a special function: when AHL is present, it forms a LuxR-AHL complex which acts on the learning subsystem (more on this later). At the moment, we will consider that AHL is absent and therefore LuxR cannot act on any subsystems. The TetR and lacI parts behave similarly: more specifically, the tetR protein in the absence of aTc inhibits the production of p22cII and LacI in the absence of IPTG inhibits the production of cI. When aTc is present, however, the p22cII production is no longer inhibited (and thus aTc is produced). Respectively, cI is produced when IPTG is present.</p> | ||
| - | === | + | === Learning subsystem === |
<p>The learning subsystem is a toggle switch with two operator sites. The upper part of the toggle (cI production) has operator sites for the LuxR-AHL complex and p22cII (which has been in turn induced by aTc). The LuxR-AHL complex induces the cI production, whereas p22cII inhibits it. The lower part of the toggle (p22cII production) has operator sites for the LuxR-AHL comple and cI (which has been induced by IPTG). Similarly with the upper part, the LuxR-AHL complex induces the p22cII production and cI inhibits it. Therefore, the switch always requires the presence of the LuxR-AHL complex in order for it to operate. Its state depends on the presence of p22cII and cI into the system, which in curse was caused through the exposure of the system to aTc and IPTG.</p> | <p>The learning subsystem is a toggle switch with two operator sites. The upper part of the toggle (cI production) has operator sites for the LuxR-AHL complex and p22cII (which has been in turn induced by aTc). The LuxR-AHL complex induces the cI production, whereas p22cII inhibits it. The lower part of the toggle (p22cII production) has operator sites for the LuxR-AHL comple and cI (which has been induced by IPTG). Similarly with the upper part, the LuxR-AHL complex induces the p22cII production and cI inhibits it. Therefore, the switch always requires the presence of the LuxR-AHL complex in order for it to operate. Its state depends on the presence of p22cII and cI into the system, which in curse was caused through the exposure of the system to aTc and IPTG.</p> | ||
| - | === | + | === Reporting subsystem === |
<p>There are four reporters in the system. CFP and YFP are active during the training phase of the system and show which chemical the system is exposed to during training, whereas GFP and RFP are active during the testing phase and show if the system is exposed to the same chemical as in training or not. | <p>There are four reporters in the system. CFP and YFP are active during the training phase of the system and show which chemical the system is exposed to during training, whereas GFP and RFP are active during the testing phase and show if the system is exposed to the same chemical as in training or not. | ||
| Line 31: | Line 31: | ||
The GFP production is regulated with help of two operator sites controlled by lacI and .</p><br> | The GFP production is regulated with help of two operator sites controlled by lacI and .</p><br> | ||
| - | == | + | == System phases == |
<p>The system operation is divided into two main phases: the training phase and the testing phase. The training phase itself is also subdivided into two phases: seeing and memorizing. During seeing, the system is first exposed to one of the two chemicals it is designed to recognize (aTc and IPTG). AHL is then added and the system’s internal toggle switch reaches a steady state. During memorizing, the chemical used during seeing is removed and only AHL is retained. This maintains the toggle switch to its acquired steady state, which is reported with YFP (if aTc was seen) or CFP (if IPTG was seen). During the testing phase, the system is exposed to any of the two chemicals (aTc or IPTG), with AHL present. By comparing its toggle switch state with the effect of the newly introduced chemical, the system shows a different response if it has previously been exposed to this chemical and reports with the same XFP as in the training phase (YFP for aTc, CFP for IPTG) or if it recognizes a different chemical and reports with a different XFP (GFP for trained with aTc and recognizing IPTG, RFP for trained with IPTG and recognizing aTc).The following table presents all possible paths that may be taken by the system during all phases of operation according to the external stimuli. </p> | <p>The system operation is divided into two main phases: the training phase and the testing phase. The training phase itself is also subdivided into two phases: seeing and memorizing. During seeing, the system is first exposed to one of the two chemicals it is designed to recognize (aTc and IPTG). AHL is then added and the system’s internal toggle switch reaches a steady state. During memorizing, the chemical used during seeing is removed and only AHL is retained. This maintains the toggle switch to its acquired steady state, which is reported with YFP (if aTc was seen) or CFP (if IPTG was seen). During the testing phase, the system is exposed to any of the two chemicals (aTc or IPTG), with AHL present. By comparing its toggle switch state with the effect of the newly introduced chemical, the system shows a different response if it has previously been exposed to this chemical and reports with the same XFP as in the training phase (YFP for aTc, CFP for IPTG) or if it recognizes a different chemical and reports with a different XFP (GFP for trained with aTc and recognizing IPTG, RFP for trained with IPTG and recognizing aTc).The following table presents all possible paths that may be taken by the system during all phases of operation according to the external stimuli. </p> | ||
| Line 151: | Line 151: | ||
|} | |} | ||
| - | === | + | === Further thoughts on the system phases === |
| - | TODO: put Stefan's new text here. Text should be | + | TODO: put Stefan's new text here. Text should be small. |
| - | |||
| - | + | == System parts == | |
| - | + | EducatETH <i>E.coli</i> consists of 11 parts that can be synthesized independently (want to know how this is done in the lab? Then visit our [https://2007.igem.org/ETHZ/Biology/Lab In the Lab] page!) Please note that four of them (4,5 and 8,9) form together two functional system units. They have been separated to ensure comparable part lengths and thus enable easier introduction into plasmids. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
{| class="wikitable" border="1" cellspacing="0" cellpadding="2" style="text-align:center; margin: 1em 1em 1em 0; background: #f9f9f9; border: 1px #aaa solid; border-collapse: collapse;" | {| class="wikitable" border="1" cellspacing="0" cellpadding="2" style="text-align:center; margin: 1em 1em 1em 0; background: #f9f9f9; border: 1px #aaa solid; border-collapse: collapse;" | ||
| Line 359: | Line 344: | ||
<br> | <br> | ||
| - | == | + | == References == |
| - | + | ||
| - | |||
| - | ===== | + | == To Do == |
| + | |||
| + | === New === | ||
<p><ul> | <p><ul> | ||
<li> Update and correct parts in parts list. Write better in a table | <li> Update and correct parts in parts list. Write better in a table | ||
| Line 379: | Line 364: | ||
<li> Make "In the lab page", replace links. | <li> Make "In the lab page", replace links. | ||
<li> Put image with 11 system parts (updated one, created by katerina) | <li> Put image with 11 system parts (updated one, created by katerina) | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
</ul></p><br> | </ul></p><br> | ||
Revision as of 08:17, 20 October 2007

In this page, you can find an analysis of the function of our system and its relation to epigenetics, its biological design and a list of the parts that it consists of. Are you interested in constructing educatETH E.coli in your lab? Then under In the Lab, you can find the ingredients and equipment we used, the electronic version of our lab notebook and a presentation of all the difficulties that we encountered. If you are also interested on how educatETH E.coli was simulated outside the lab, please visit the Engineering Perspective.
Contents |
Introduction
educatETH E.coli is a system which can distinguish between aTc and IPTG based on a previous training phase conducted with the same chemicals and the help of AHL. It composes of three subsystems: the subsystem of constitutively produced proteins, the learning subsystem and the reporting subsystem. The constitutively produced proteins (lacI, TetR and LuxR) control the learning subsystem. At the core of the latter there exists a modified version of the toggle switch found in [1] with two operator sites, so that it only changes its state when both one of the two chemicals (aTc/IPTG) and AHL are present. As AHL is only present during the training phase, the toggle maintains its state during testing, and thus can “memorize”. In the reporting subsystem, four reporters allow supervision of both the chemical the system was trained with and of if the system recognizes the chemical it is being exposed to in the testing phase as one it has been trained with or not.
The complete system
The biological design of EducatETH E.coli is presented on
. In the following, we will clarify the function of all depicted components. (Are you interested in how the complex system of Fig. 1 was modelled? Then visit the Engineering Perspective!)Constitutive subsystem
The constitutively produced proteins of the system are LacI, TetR and LuxR. The LuxR part has a special function: when AHL is present, it forms a LuxR-AHL complex which acts on the learning subsystem (more on this later). At the moment, we will consider that AHL is absent and therefore LuxR cannot act on any subsystems. The TetR and lacI parts behave similarly: more specifically, the tetR protein in the absence of aTc inhibits the production of p22cII and LacI in the absence of IPTG inhibits the production of cI. When aTc is present, however, the p22cII production is no longer inhibited (and thus aTc is produced). Respectively, cI is produced when IPTG is present.
Learning subsystem
The learning subsystem is a toggle switch with two operator sites. The upper part of the toggle (cI production) has operator sites for the LuxR-AHL complex and p22cII (which has been in turn induced by aTc). The LuxR-AHL complex induces the cI production, whereas p22cII inhibits it. The lower part of the toggle (p22cII production) has operator sites for the LuxR-AHL comple and cI (which has been induced by IPTG). Similarly with the upper part, the LuxR-AHL complex induces the p22cII production and cI inhibits it. Therefore, the switch always requires the presence of the LuxR-AHL complex in order for it to operate. Its state depends on the presence of p22cII and cI into the system, which in curse was caused through the exposure of the system to aTc and IPTG.
Reporting subsystem
There are four reporters in the system. CFP and YFP are active during the training phase of the system and show which chemical the system is exposed to during training, whereas GFP and RFP are active during the testing phase and show if the system is exposed to the same chemical as in training or not. More specifically, the YFP protein production is regulated with help of two operator sites controlled by cI and aTc. cI inhibits YFP production and aTc induces it. Therefore, YFP is produced when the system is exposed to aTc. In a similar manner, the CFP production is produced when the system is exposed to IPTG. The GFP production is regulated with help of two operator sites controlled by lacI and .
System phases
The system operation is divided into two main phases: the training phase and the testing phase. The training phase itself is also subdivided into two phases: seeing and memorizing. During seeing, the system is first exposed to one of the two chemicals it is designed to recognize (aTc and IPTG). AHL is then added and the system’s internal toggle switch reaches a steady state. During memorizing, the chemical used during seeing is removed and only AHL is retained. This maintains the toggle switch to its acquired steady state, which is reported with YFP (if aTc was seen) or CFP (if IPTG was seen). During the testing phase, the system is exposed to any of the two chemicals (aTc or IPTG), with AHL present. By comparing its toggle switch state with the effect of the newly introduced chemical, the system shows a different response if it has previously been exposed to this chemical and reports with the same XFP as in the training phase (YFP for aTc, CFP for IPTG) or if it recognizes a different chemical and reports with a different XFP (GFP for trained with aTc and recognizing IPTG, RFP for trained with IPTG and recognizing aTc).The following table presents all possible paths that may be taken by the system during all phases of operation according to the external stimuli.
| aTc | IPTG | AHL | p22cII | cI | Reporting | |
|---|---|---|---|---|---|---|
| Start | no | no | no | no | no | no |
| Learning | ||||||
| Seeing | ||||||
| Trained with aTc | yes | no | no | yes | no | yfp |
| Trained with IPTG | no | yes | no | no | yes | cfp |
| Memorizing | ||||||
| Trained with aTc | yes | no | yes | yes | no | yfp |
| Trained with IPTG | no | yes | yes | no | yes | cfp |
| Testing | ||||||
| Trained with aTc Tested with aTc | yes | no | yes | yes | no | yfp |
| Trained with aTc Tested with IPTG | no | yes | yes | yes | no | gfp |
| Trained with IPTG Tested with IPTG | no | yes | yes | no | yes | cfp |
| Trained with IPTG Tested with aTc | yes | no | yes | no | yes | rfp |
Further thoughts on the system phases
TODO: put Stefan's new text here. Text should be small.
System parts
EducatETH E.coli consists of 11 parts that can be synthesized independently (want to know how this is done in the lab? Then visit our In the Lab page!) Please note that four of them (4,5 and 8,9) form together two functional system units. They have been separated to ensure comparable part lengths and thus enable easier introduction into plasmids.
| 1 | TetR production | [http://partsregistry.org/Part:BBa_I739001 BBa_I739001] | constitutive subsystem | |
|---|---|---|---|---|
| 2 | LacI production | [http://partsregistry.org/Part:BBa_I739002 BBa_I739002] | constitutive subsystem | |
| 3 | LuxR production | [http://partsregistry.org/Part:BBa_I739003 BBa_I739003] | constitutive subsystem | |
| 4 | 1st half of P22 cII / EYFP production | [http://partsregistry.org/Part:BBa_I739004 BBa_I739004] | reporting subsystem | |
| 5 | 2nd half of P22 cII / EYFP production | [http://partsregistry.org/Part:BBa_I739005 BBa_I739005] | reporting subsystem | |
| 6 | cI production | [http://partsregistry.org/Part:BBa_I739006 BBa_I739006] | learning subsystem | |
| 7 | P22 cII production | [http://partsregistry.org/Part:BBa_I739007 BBa_I739007] | learning subsystem | |
| 8 | 1st half of cI / ECFP production | [http://partsregistry.org/Part:BBa_I739008 BBa_I739008] | reporting subsystem | |
| 9 | 2nd half of cI / ECFP production | [http://partsregistry.org/Part:BBa_I739009 BBa_I739009] | reporting subsystem | |
| 10 | RFP production | [http://partsregistry.org/Part:BBa_I739010 BBa_I739010] | reporting subsystem | |
| 11 | GFP production | [http://partsregistry.org/Part:BBa_I739011 BBa_I739011] | reporting subsystem |
| 1+2+3 | tetR + lacI + luxR production | [http://partsregistry.org/Part:BBa_I739013 BBa_I739013] | constitutive subsystem | |
|---|---|---|---|---|
| 4+5 | P22 cII + EYFP production | [http://partsregistry.org/Part:BBa_I739015 BBa_I739015] | reporting subsystem | |
| 8+9 | cI + ECFP production | [http://partsregistry.org/Part:BBa_I739016 BBa_I739016] | reporting subsystem | |
| (4+5)+(8+9) | (P22 cII + EYFP) + (cI + ECFP) production | [http://partsregistry.org/Part:BBa_I739017 BBa_I739017] | reporting subsystem | |
| 6+7 | cI + P22 cII production | [http://partsregistry.org/Part:BBa_I739018 BBa_I739018] | learning subsystem | |
| 10+11 | RFP + GFP production | [http://partsregistry.org/Part:BBa_I739019 BBa_I739019] | reporting subsystem | |
| (6+7)+(10+11) | (cI + P22 cII) + (RFP + GFP) production | [http://partsregistry.org/Part:BBa_I739020 BBa_I739020] | learning/reporting subsystem |
| 1' | cI negative / tetR negative promoter | [http://partsregistry.org/Part:BBa_I739102 BBa_I739102] | reporting subsystem | |
|---|---|---|---|---|
| 2' | lacI negative / P22 cII negative promoter | [http://partsregistry.org/Part:BBa_I739103 BBa_I739103] | reporting subsystem | |
| 3' | luxR/HSL positive / P22 cII negative promoter | [http://partsregistry.org/Part:BBa_I739104 BBa_I739104] | learning subsystem | |
| 4' | luxR/HSL positive / cI negative promoter | [http://partsregistry.org/Part:BBa_I739105 BBa_I739105] | learning subsystem | |
| 5' | tetR negative / P22 cII negative promoter | [http://partsregistry.org/Part:BBa_I739106 BBa_I739106] | reporting subsystem | |
| 6' | cI negative / lacI negative promoter | [http://partsregistry.org/Part:BBa_I739107 BBa_I739107] | reporting subsystem |
| 1" | PoC promoter | [http://partsregistry.org/Part:BBa_I739101 BBa_I739101] | proof of concept, no part of the system | |
|---|---|---|---|---|
| 2" | PoC intermediate | [http://partsregistry.org/Part:BBa_I739014 BBa_I739014] | proof of concept, no part of the system | |
| 3" | PoC composite | [http://partsregistry.org/Part:BBa_I739021 BBa_I739021] | proof of concept, no part of the system |
References
To Do
New
- Update and correct parts in parts list. Write better in a table
- Update and correct full system scheme
- Update graph scheme (made by Stefan) using aTc, IPTG instead of it1,2 and ia1,2
- Which reporters are active when? I think CFP and YFP are not active only during training. Change text if needed.
- Proposed terminology: seeing, memorizing
- What are GFP, RFP controlled by? Is the full system scheme correct there?
- What are the “double promoters” mentioned?
- Check my terminology (operator sites etc)
- Put Stefan's updated part on epigenetics
- How was Sven’s standard notation on how to write differently proteins, dna, rna?
- Fill in table completely, make it more reading-friendly
- Make "In the lab page", replace links.
- Put image with 11 system parts (updated one, created by katerina)