ETHZ/Simulation
From 2007.igem.org
(→Simulating the EducatETH <i>E. coli</i> System) |
(→Simulation of Test Cases) |
||
| Line 12: | Line 12: | ||
== Simulation of Test Cases == | == Simulation of Test Cases == | ||
| - | To test | + | To test all the possible conditions and transitions in our system, we run timing simulations for all 4 different branches in the flow diagram of our protocol, and we plot the concentrations of the inducers, memory proteins and reporter proteins. During each learning, memorizing and recognizing phase, we wait for the steady state of the system to be reached. The three phases of the system are thus separated sufficiently in time. In all the cases, we follow the following procedure: |
| - | * During the first 1000 minutes of the simulation we | + | * During the first 1000 minutes of the simulation, we do not add any inducers to the system, and as a result, we check for the baseline production of proteins. |
| - | * Between 1000 and 2000 minutes we | + | * Between 1000 and 2000 minutes, we add the inducer that should be learned, and we let the system reach steady state. |
| - | * After the 2000<sup>th</sup> minute we | + | * After the 2000<sup>th</sup> minute, we add the inducer AHL for memory formation, and run the simulation for an additional 1000 minutes, to reach again steady state. |
| - | * Between 3000 and 4000 minutes we | + | * Between 3000 and 4000 minutes, we tested the final behavior of the system, and we check if it reacts in the desired way, by showing the appropriate color. |
| - | + | The parameters of the system are crucial if one wants to have accurate and realistic simulations. We present the parameters that we used to simulate our system, in the section [[ETHZ/Parameters | Parameters]]. | |
| - | + | === Test case 1: Learn and recognize IPTG === | |
[[Image:ETHZTest1.png|center|thumb|Fig. 2: Learn and recognize IPTG |720px]] | [[Image:ETHZTest1.png|center|thumb|Fig. 2: Learn and recognize IPTG |720px]] | ||
Revision as of 11:38, 21 October 2007

Simulating the EducatETH E. coli System
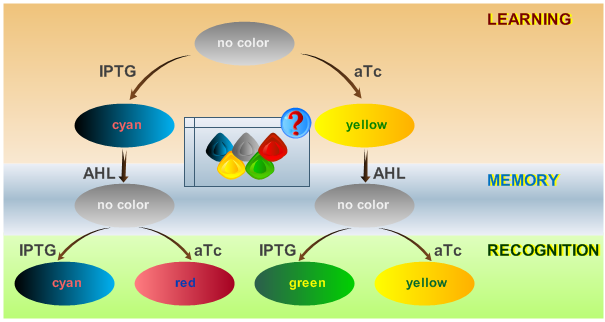
As has been presented in the Modeling page, we have created a model of our system, that can be described as a finite state machine. In order to examine our system's behavior thoroughly, we have to simulate it through all the difference phases that can be reached. We carry out our simulations based on the protocol presented in Fig. 1.
Simulation of Test Cases
To test all the possible conditions and transitions in our system, we run timing simulations for all 4 different branches in the flow diagram of our protocol, and we plot the concentrations of the inducers, memory proteins and reporter proteins. During each learning, memorizing and recognizing phase, we wait for the steady state of the system to be reached. The three phases of the system are thus separated sufficiently in time. In all the cases, we follow the following procedure:
- During the first 1000 minutes of the simulation, we do not add any inducers to the system, and as a result, we check for the baseline production of proteins.
- Between 1000 and 2000 minutes, we add the inducer that should be learned, and we let the system reach steady state.
- After the 2000th minute, we add the inducer AHL for memory formation, and run the simulation for an additional 1000 minutes, to reach again steady state.
- Between 3000 and 4000 minutes, we tested the final behavior of the system, and we check if it reacts in the desired way, by showing the appropriate color.
The parameters of the system are crucial if one wants to have accurate and realistic simulations. We present the parameters that we used to simulate our system, in the section Parameters.
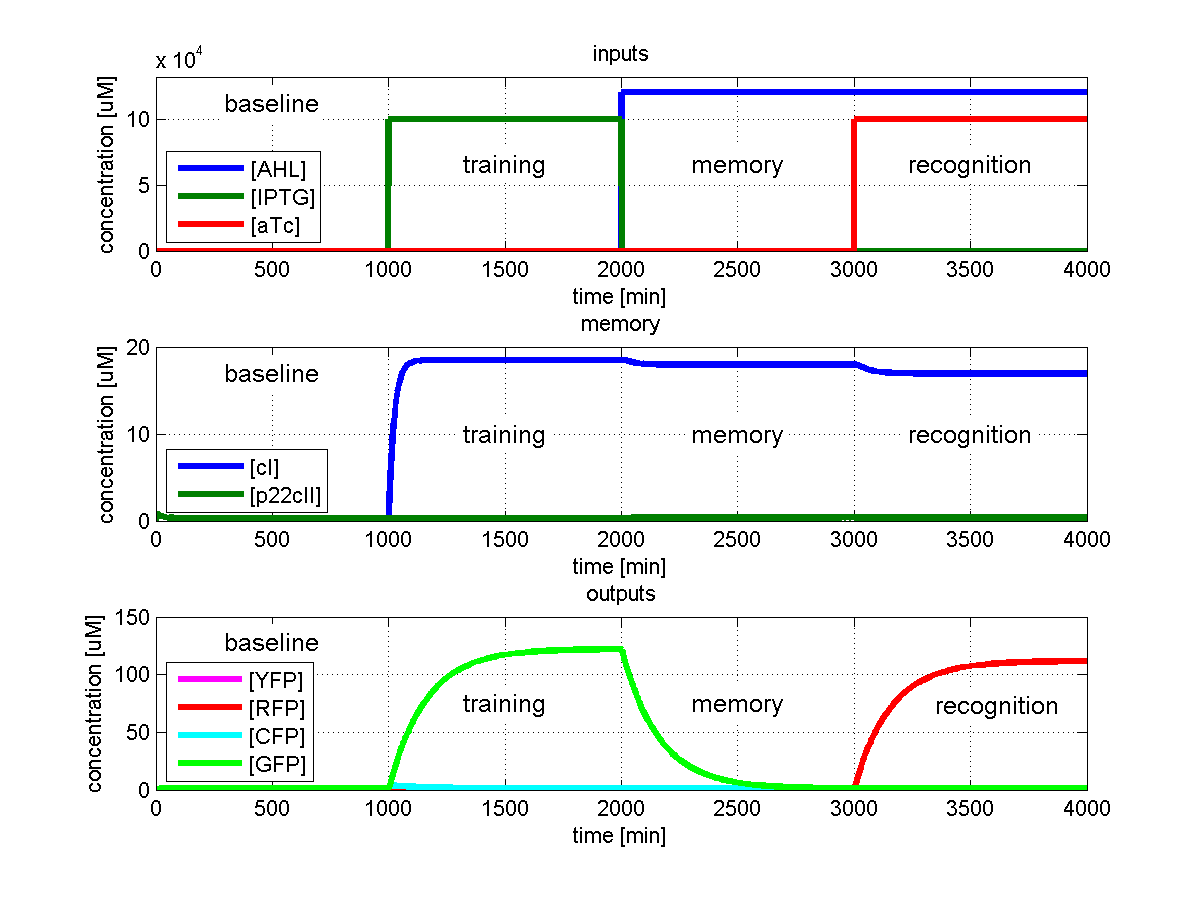
Test case 1: Learn and recognize IPTG
Fig. 2 shows the simulated behavior of the system when IPTG is presented both during the learing phase and the recognition phase. In both the learning and recognition phase the system reports by producing green florescence proteins which matches the desired behavior.
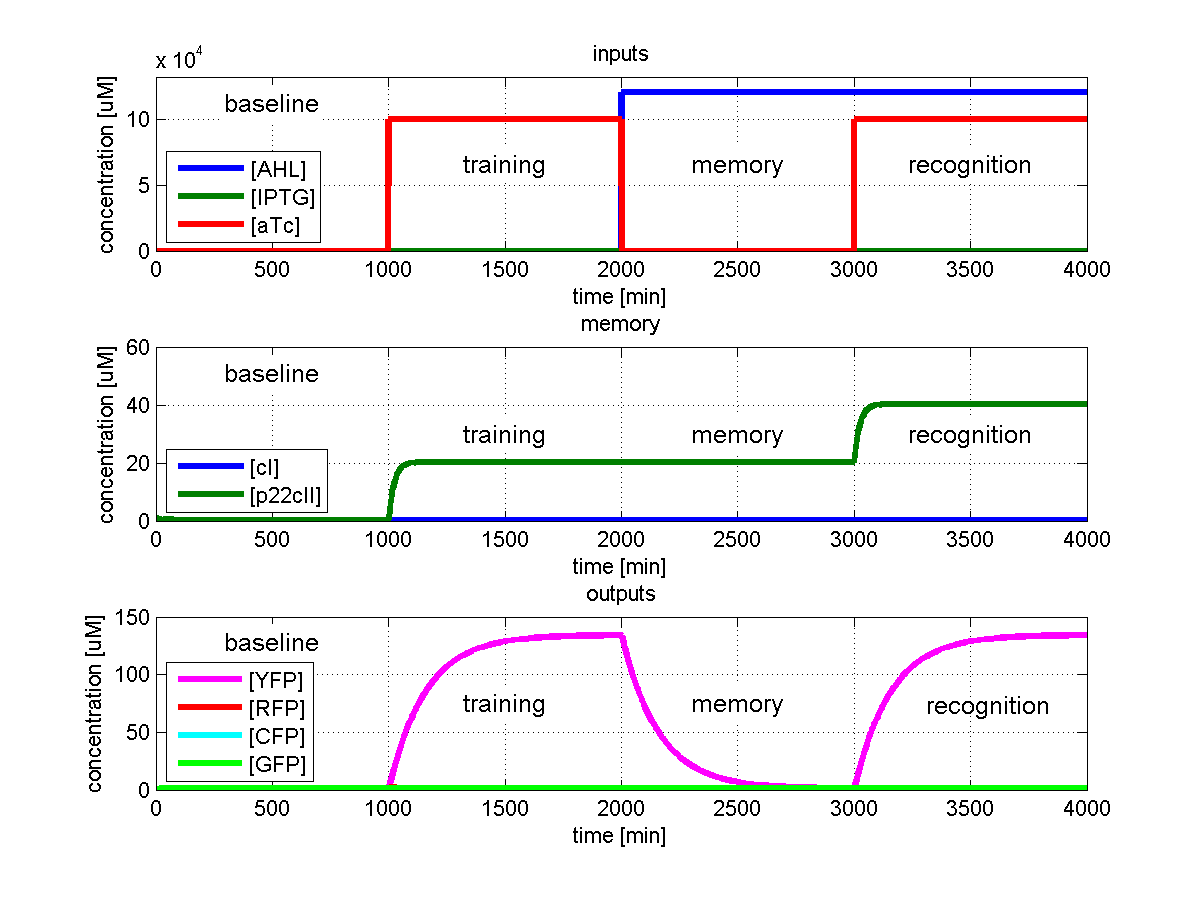
Test 2: Learn IPTG and get tested for aTc
Fig. 3 shows the simulated behavior of the system when IPTG is presented only during the learing phase but aTc during the recognition phase. In contrast to Fig. 2 the system reports by producing red florescence proteins during the recognition phase which matches the desired behavior.
Test 3: Learn and recognize aTc
Fig. 4 shows the simulated behavior of the system when aTc is presented both during the learing phase and the recognition phase. In both the learning and recognition phase the system reports by producing yellow florescence proteins which matches the desired behavior.
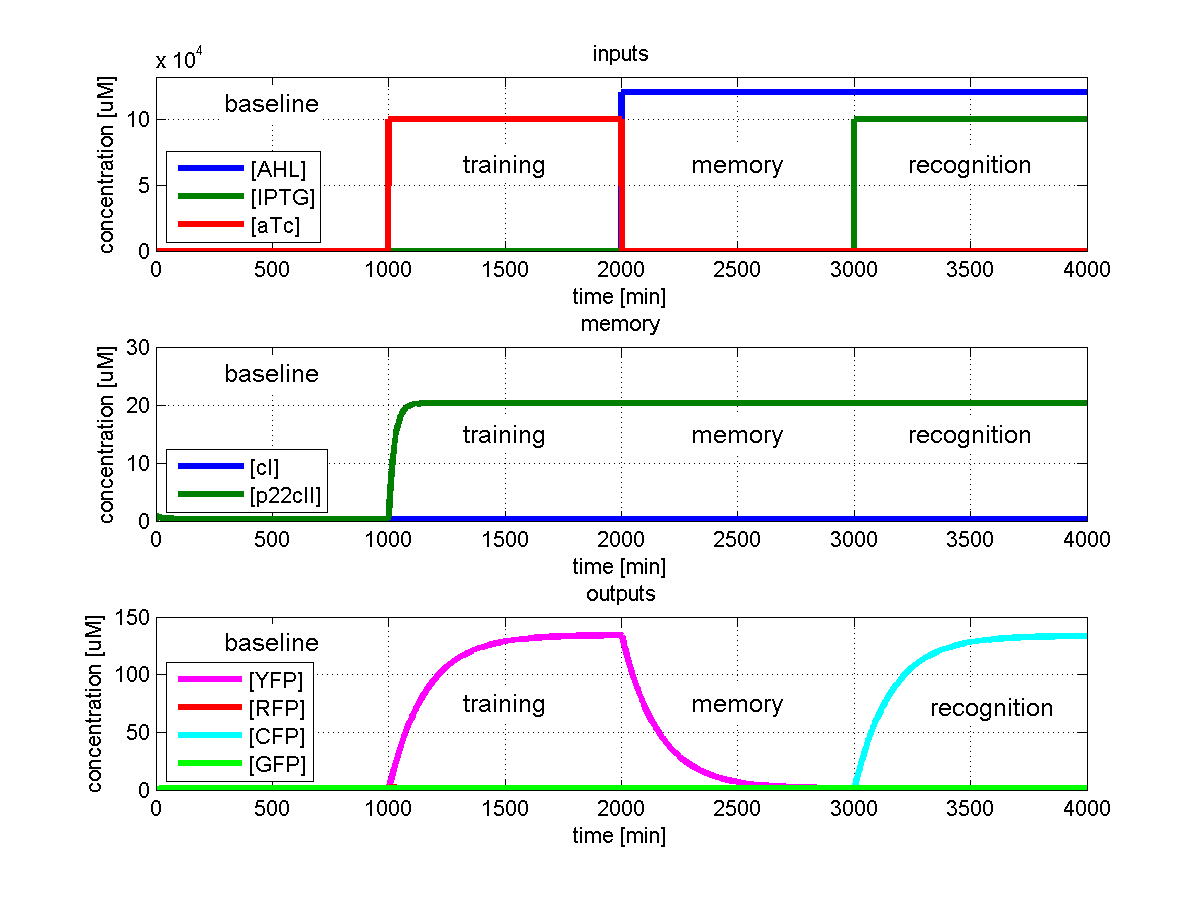
Test 4: Learn aTc and get tested for IPTG
Fig. 5 shows the simulated behavior of the system when aTc is presented only during the learing phase but IPTG during the recognition phase. In contrast to Fig. 4 the system reports by producing cyan florescence proteins during the recognition phase which matches the desired behavior.
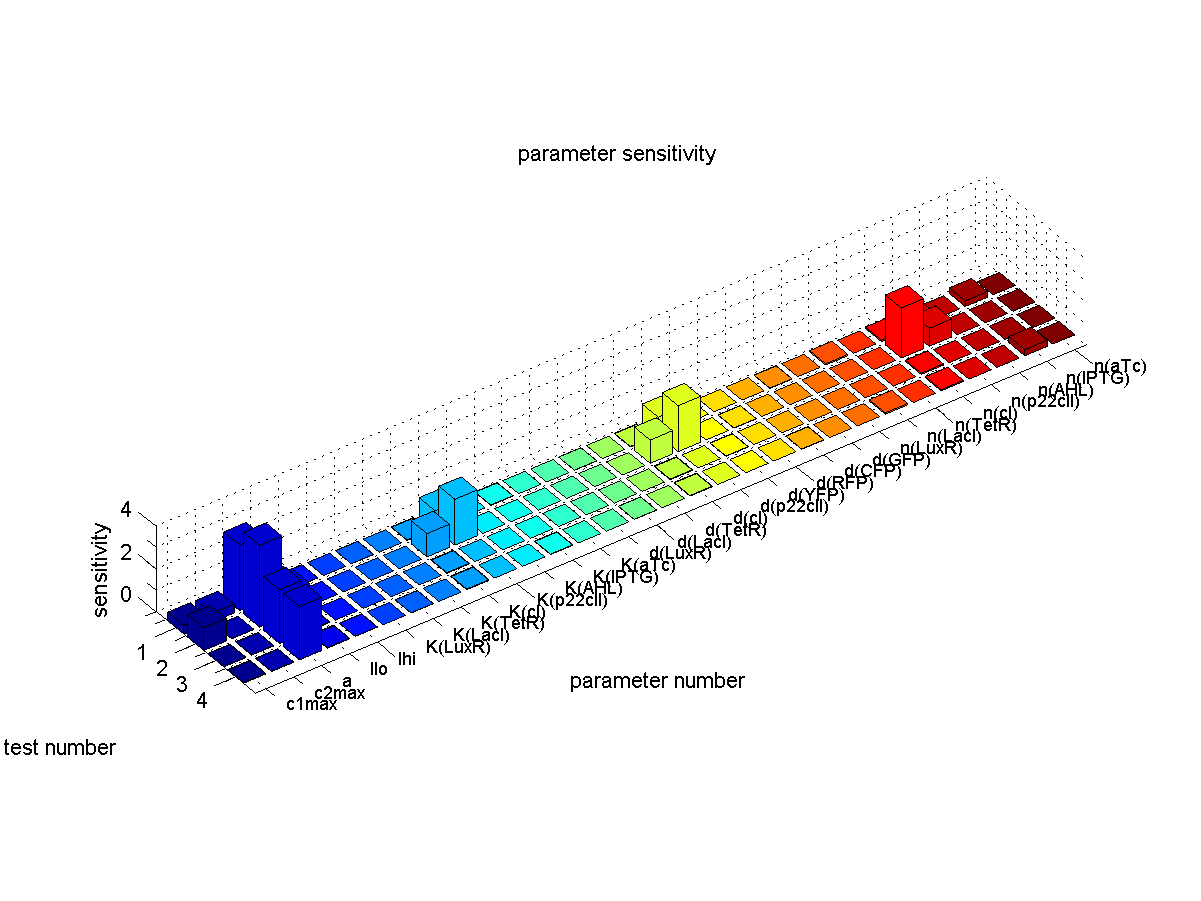
Sensitivity Analysis
We were not only interested in finding a set of parameters for which our system is working. To provide a stable performance also in the presence of noise and parameter missmatch we were interested in analysing the sensitivity of the system to find the most sensitive parameters.
There for we simulated the timing behavior while sweeping the parameters by 5%. In detail we did the following for e.g. test case 1: For each paramter we did the timing simulation like shown in Fig. 2 once while increasing the paramter by 5% and once while decreasing the parameter by 5%. Since in test case 1 we are interested in having a high concentration of GFP during the recognition phase with respect to the base line we divide the steady state GFP concentration at 4000 minutes by the baseline concentration at 1000 minutes. Finally as shown in the following equation we define the sensitivity by subtracting the resulting factor that we get with an increase parameter from the resulting factor that we get by decreasing the parameter.
So if there is no difference in the concentration of the desired output during the recognition phase with respect to baseline when sweeping a parameter by +/-5% the sensitivity of that parameter is equal to zero. Otherwise the sensitivity gives us a measure of how much the concentration changes.
We quantified the sensitivity for all parameters and test cases. The results are plotted in Fig. 5.
We found the following results:
- Our system is most sensitve to the parameter a which describes the base production. This is not a surpising result since we depend on having a good switch and our signal to noise or with other words output to baseline production level highly depends on the fact that we can reliably switch off the production of the memory proteins cI and p22cII. For a more detailed analysis of the switching behavior please see ADD LINK TO ANALYSIS!!!
- Other sensitive parameters are those that are directly related to the production and decay of the memory proteins cI and p22cII. This can be explained by the fact that our system prefers symmetry between the parameter sets of cI and p22cII to support good switching behavior where the same concentration of p22cII leads to the same repression of cI than vice versa. We can especially see that those parameters seem to be more sensitive for the test cases 1 and 2 where cI has to be stored inside the memory. The reason for this behavior is that due to the missing symmetry between the parameters for cI and p22cII we currently have a bias inside the system towards the production of p22cII.