Imperial/Wet Lab/Protocols/ID2.2
From 2007.igem.org
(Difference between revisions)
Revision as of 02:38, 22 October 2007
Aims:
The system was tested for AHL sensitivity with concentrations in the range of real biofilms, with AHL concentrations ranging from as low as 0.1nM to 50nM.
Contents |
Equipments
- Fluorometer + PC
- 25°C water bath
- 1 Fluorometer plates (black)
- 1 Sealing plate mats
- Gilson pipettes 200, 20, 10
- Eppendorf Tube x 4
- Plate Centrifuge
- Stopwatch
Reagents
- Commercial S30 E.coli extract. Including:
- 175µl Amino Acid Mixture Minus Cysteine, 1mM
- 175µl Amino Acid Mixture Minus Methionine, 1mM
- 175µl Amino Acid Mixture Minus Leucine, 1mM
- 450µl S30 Extract, Circular (3 × 150µl)
- 750µl S30 Premix Without Amino Acids
- Nuclease Free water
- AHL solutions C
- DNA construct
Protocols
- We will test for more values between the 1nM to 10nM [AHL] range, at concentrations of 3nM, 5nM and 7nM.
- Label three eppendorf tubes C1, C2 and C3.
- Using solution C, add 3ul, 5ul and 7ul into tubes C1, 2, and 3 respectively.
- Add 7ul, 5ul and 3ul of nuclease free water into tubes C1, 2, and 3 respectively. This forms the [AHL] concentrations for making the 3nM, 5nM and 7nM dilutions for testing.
- Then collect all equipment and reagents and ensure that the fluorometer and that the PC connected has a data collection protocol installed.
- Place the 96 well plates together with their plate mates in their respective incubators so as to heat them up to the appropriate temperature before the experiments start.
- For the next step of the go to the biochemistry level 5 and remove:
- A.A's from kits
- Premix tubes (200ul)
- S30 tubes (150ul)
- For each AHL concentration Tested Prepare the following
- Commercial E.coli Cell Extract: First prepare a complete amino acid mixture for both extract solutions: Add the 25.0μl volume of two amino acid minus mixtures into an labeled eppendorf to give a volume of 35μl. Each amino acid minus mixture is missing one type of amino acid, and so by combining two solutions we are complementing each solution for the missing amino acid. Place eppendorf in a rack on bench.
- Commercial E.coli Cell Extract:Add all of the E.coli complete amino acid mixture to S30 Premix Without Amino Acid and S30 Extract Circular DNA. Place the eppendorf tube in a rack on the bench.
- Vortex the tubes to mix thoroughly and place 40ul into each well.
- Place 20ul of midipreped DNA plasmid into each of the filled wells.
- Any left over premix or cell extract should be returned to the freezer in biochemistry level 5 and labeled with new volumes.
Schematic
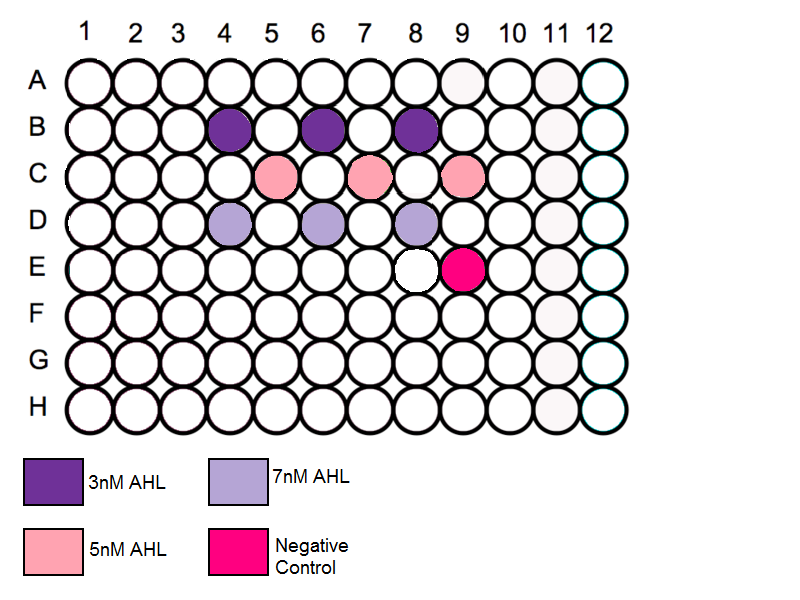
- Throughout our series of experiments we are reusing the 96 well plates. It is important to measure each plate before the schematic is determined, this is to prevent any contamination.
- Using the protocol above using wells near the edge should be avoided and the wells spread out throughout the plate
- Add 3ul of solution C1 to three wells . [AHL]=3nM
- Add 3ul of solution C2 to three wells . [AHL]=5nM
- Add 3ul of solution C3 to three wells . [AHL]=7nM
- Add 3ul of nuclease free water to well E8. [AHL]=0nM
Loading Plate
- Follow the schematic for the plate and begin by loading the in vitro expression system into the correct wells. Before loading in the samples vortex the tubes for a few seconds to mix the solution.
- Tap down the top of the lid to bring down any solution to bottom of the well.
- Remove lid off the 96 well plate and place in the fluorometer. Create a file name insert temp under: D:\IGEM\INSERT DATE\ID\ 25oC. Export the data here. Each file should be named as the following:
- construct-temp-time-date
- This measurement will give a back ground fluorescence measurement and can be used as our time zero data.
- Then to begin the reaction add 20μl of purified DNA sample to each well indicated on the schematic. Be careful not to add to wells that DO NOT NEED DNA.
- Place lid back on and place back in the respective incubators.
- After 10 minutes of incubation measure the fluorescence by repeating procedure 3-4 above. This initial measurement of 10 minutes should be carried on for 1 hours or until it appears GFP production levels off.
- Before each measurement be careful to remember to tap down the solution and to remove the lid before placing in the fluorometer.
|