Freiburg07/report Ca sensor
From 2007.igem.org
m |
m |
||
| Line 55: | Line 55: | ||
In-Vivo-Analysis:<br> | In-Vivo-Analysis:<br> | ||
As DHFR is essential for bacterial growth in minimal media with trimethioprim (TMP), | As DHFR is essential for bacterial growth in minimal media with trimethioprim (TMP), | ||
| - | we could show that the ability of E.colis with our plasmid to grow is directly dependant on the presence -and, furthermore, concentration- of calcium-ions (Ca2+)in the media: | + | we could show that the ability of E.colis with our plasmid to grow is directly dependant on the presence -and, furthermore, concentration- of calcium-ions (Ca2+)in the media:[https://2007.igem.org/Freiburg07/In_vivo_test_II In vivo test II] |
<h2>Discussion</h2> | <h2>Discussion</h2> | ||
Revision as of 15:21, 25 October 2007
Contents |
A single-protein calcium ion sensor mediating cell survival
Introduction
Split enzymes:
Some enzymes can be divided into two separate parts that won´t show any activity until both parts are physically brought together again;
this circumstance allows enzyme-activity-assays.
As those assays were already being used in our lab before, we could access plasmids containing enzyme-halfs of beta-lactamase and dihydrofolatereductase (DHFR).
Calmodulin:
This molecule is known for its strong conformational change upon binding calcium.
Truong, Ikura et al.(Ontario Cancer Institute and [http://medbio.utoronto.ca/ Department of medical Biophysics] , University of Toronto) have fused CFP and YFP to the ends of modified calmodulin and shown that you can then induce FRET between them by adding calcium.
This work inspired us to test calmodulin as "switch" for our split enzymes. Prof. Ikura was so friendly to send us his calmodulin (as plasmid with sequence, thanks a lot again!) so that we could gain it via PCR.
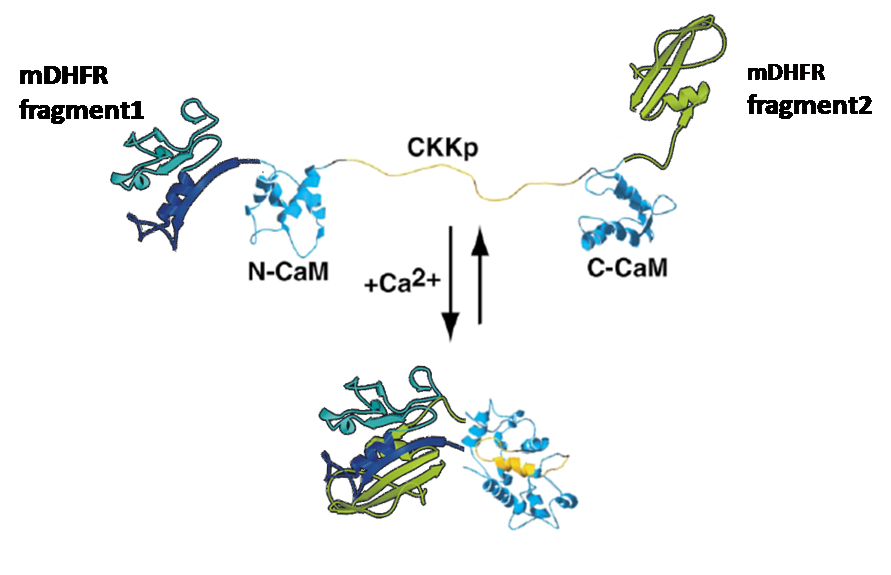
The idea:
Was to attach those enzyme-halfs to several trigger-proteins (here: calmodulin)
by cloning each one of the enzyme-halfs and the trigger-protein together in one plasmid.
This plasmid would then contain the code for the whole "protein-machinery", allowing in vivo-tests as well as expression and purification of the engineered protein, an on/off-switchable enzyme:
Materials and Methods
Planning:
-Plasmid maps, sequences, alignments and protein dates have been worked out using gcg, a unix-based, rather antiquate genetic engineering program
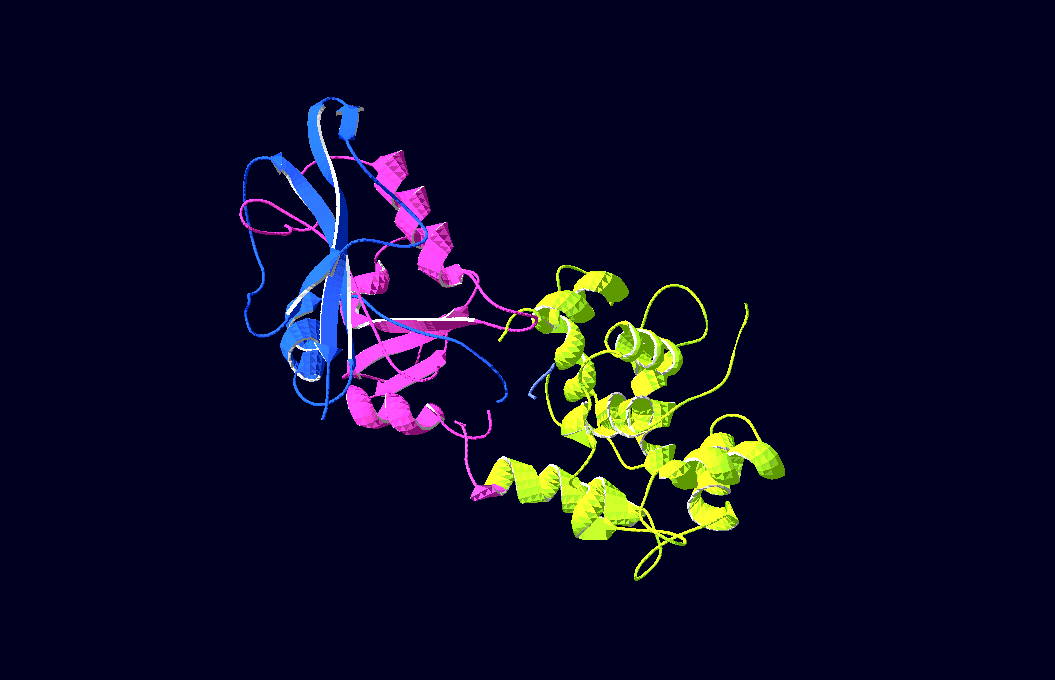
-3D-Models of our constructs (as shown in the pictures above) have been created/modified using (Mac-)Pymol and SwissPdbViewer (both freeware:[http://pymol.sourceforge.net/ Pymol],[http://expasy.org/spdbv/ SwissPdbViewer])
Clonifying:
-Clonifying steps have been done as described under protocols or with Qiagen-products
Protein expression and purification:
-Expression cultures (Volume: 2 liters) have been raised at 37 deg. C and induced by IPTG
-Protein purifications have been carried out via Ni-NTA-columns; the His-Tagged proteins have been eluted in buffers with rising imidazole-concentrations and were then analyzed on SDS-gels
Results
Plasmids:
We gained plasmids containing both parts of DHFR linked to calmoduline by 2/4/6 glycins;
in-vivo-growth tests showed the best activity for 4-glycine linkers;
thus we decided to proceed
with this linker-length.
In-Vivo-Analysis:
As DHFR is essential for bacterial growth in minimal media with trimethioprim (TMP),
we could show that the ability of E.colis with our plasmid to grow is directly dependant on the presence -and, furthermore, concentration- of calcium-ions (Ca2+)in the media:In vivo test II
Discussion
References
split DHFR:
Pelletier JN, Arndt KM, Plückthun A, Michnick SW. "An in vivo library-versus-library selection of optimized protein-protein interactions." Nat Biotechnol. 1999 Jul;17(7):683-90.
Pelletier JN, Campbell-Valois FX, Michnick SW. "Oligomerization domain-directed reassembly of active dihydrofolate reductase from rationally designed fragments." Proc Natl Acad Sci U S A. 1998 Oct 13;95(21):12141-6. [http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=9770453&ordinalpos=5&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum pubmed]
Calmodulin Sensor:
Pham E, Chiang J, Li I, Shum W, Truong K "A computational tool for designing FRET protein biosensors by rigid-body sampling of their conformational space." Structure. 2007 May;15(5):515-23.
Truong K, Sawano A, Miyawaki A, Ikura M. "Calcium indicators based on calmodulin-fluorescent protein fusions." Methods Mol Biol. 2007;352:71-82.
Truong K, Sawano A, Mizuno H, Hama H, Tong KI, Mal TK, Miyawaki A, Ikura M. "FRET-based in vivo Ca2+ imaging by a new calmodulin-GFP fusion molecule." Nat Struct Biol. 2001 Dec;8(12):1069-73.
Reviews on calmodulin: Grabarek Z "Structural basis for diversity of the EF-hand calcium-binding proteins." J Mol Biol. 2006 Jun 9;359(3):509-25.
Soderling TR, Stull JT "Structure and regulation of calcium/calmodulin-dependent protein kinases." Chem Rev. 2001 Aug;101(8):2341-52