Tianjin/FLIP-FLOP/Experiment/result
From 2007.igem.org
(Difference between revisions)
| Line 1: | Line 1: | ||
{| | {| | ||
| + | |- | ||
| + | |width="960px" style="padding: 10px; background-color: #FFFF99" | | ||
[[Image:tjuexpff201.jpg|500px]]<br> | [[Image:tjuexpff201.jpg|500px]]<br> | ||
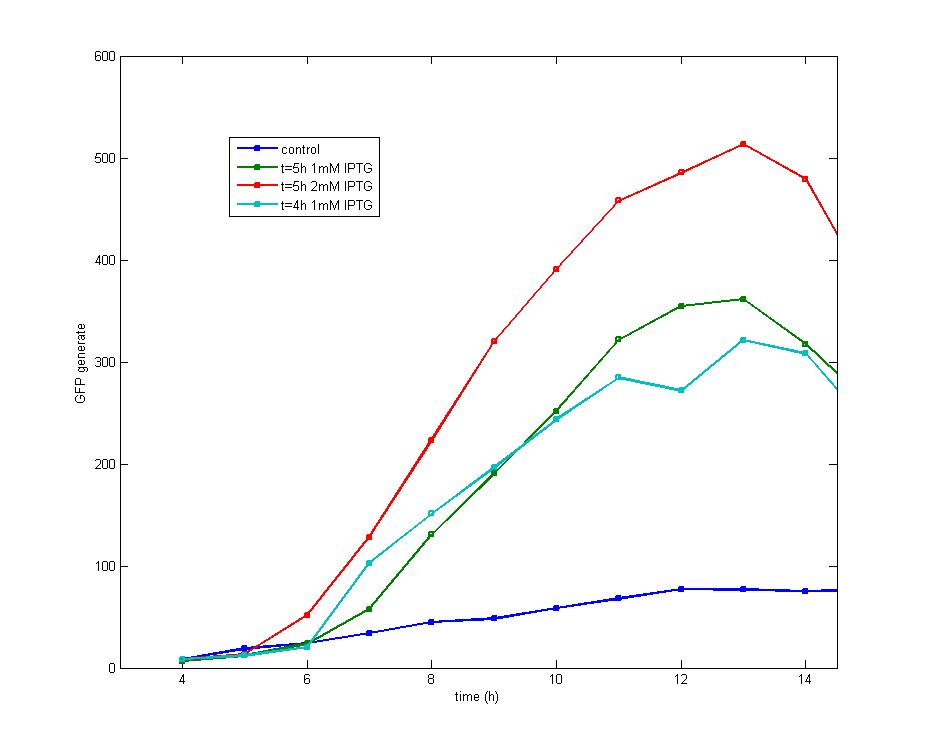
Figure 1. After the addition of 1 mM IPTG into cultures with an OD<sub>600</sub> value of 0.4(cultured for 4 hours) and 0.6(cultured for 5 hours) respectively, it is discovered that both share the similar shape of GFP fluorescence peak while early addition of IPTG could produce early peak. Addition of 2mM IPTG tends to produce a higher peak than 1mM IPTG at the OD<sub>600</sub>=0.6, but both curves share the same changing tendency.<br><br> | Figure 1. After the addition of 1 mM IPTG into cultures with an OD<sub>600</sub> value of 0.4(cultured for 4 hours) and 0.6(cultured for 5 hours) respectively, it is discovered that both share the similar shape of GFP fluorescence peak while early addition of IPTG could produce early peak. Addition of 2mM IPTG tends to produce a higher peak than 1mM IPTG at the OD<sub>600</sub>=0.6, but both curves share the same changing tendency.<br><br> | ||
| Line 10: | Line 12: | ||
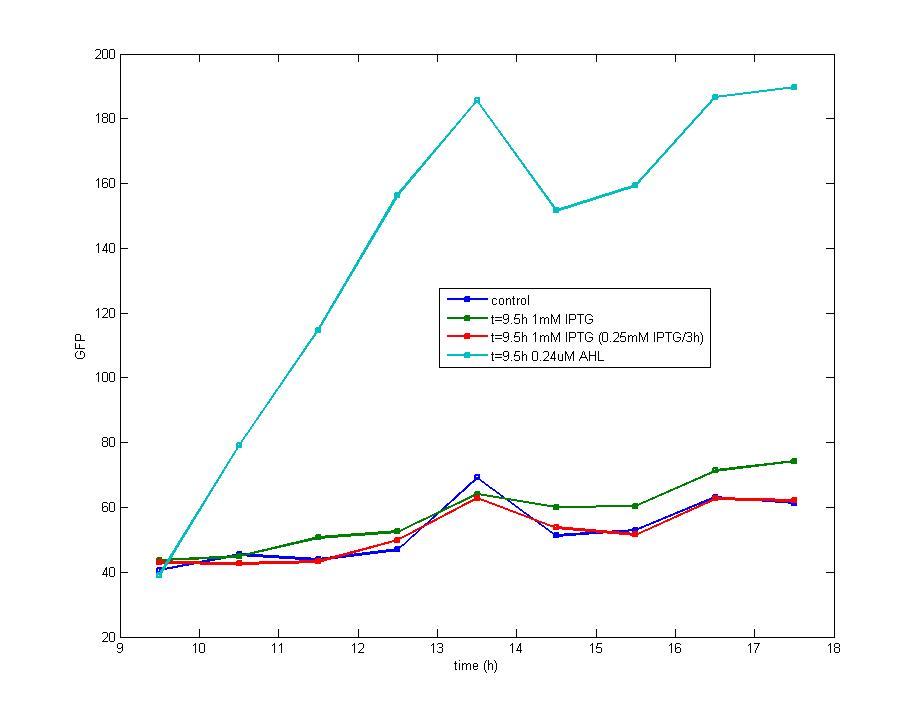
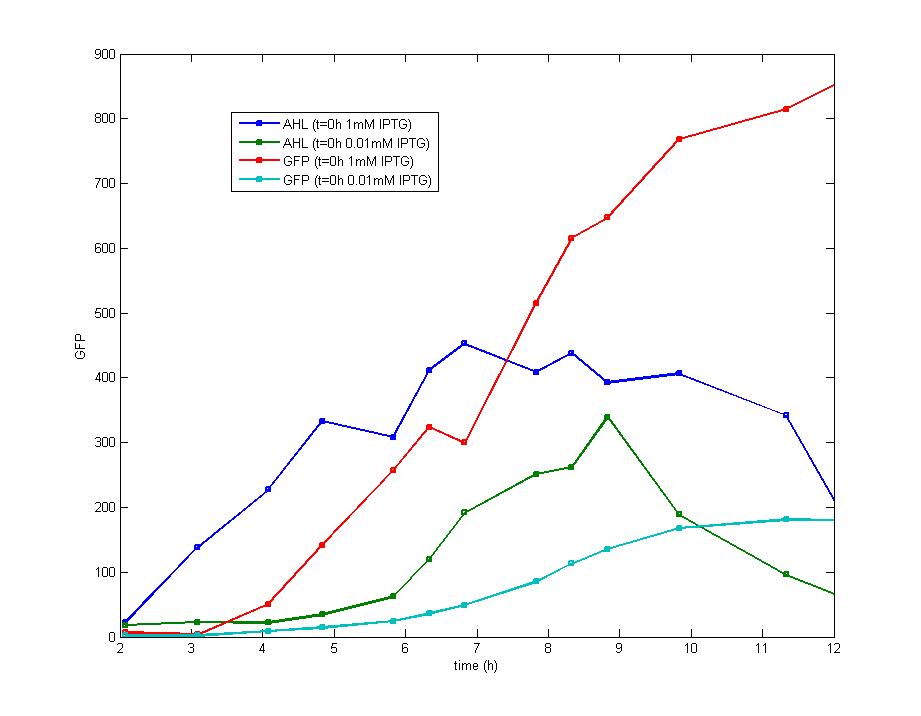
Figure 4. The concentration of extracellular AHL is examined after the induction of diluted IPTG and it seems there is no difference between the result of 0.5mM IPTG and 0.1mM IPTG. The AHL contents decreased remarkably during the stationary phase which could be attributed to the fast degradation rate and the cease of IPTG induction.<br><br> | Figure 4. The concentration of extracellular AHL is examined after the induction of diluted IPTG and it seems there is no difference between the result of 0.5mM IPTG and 0.1mM IPTG. The AHL contents decreased remarkably during the stationary phase which could be attributed to the fast degradation rate and the cease of IPTG induction.<br><br> | ||
[[Image:tjuexpff205.jpg|500px]]<br> | [[Image:tjuexpff205.jpg|500px]]<br> | ||
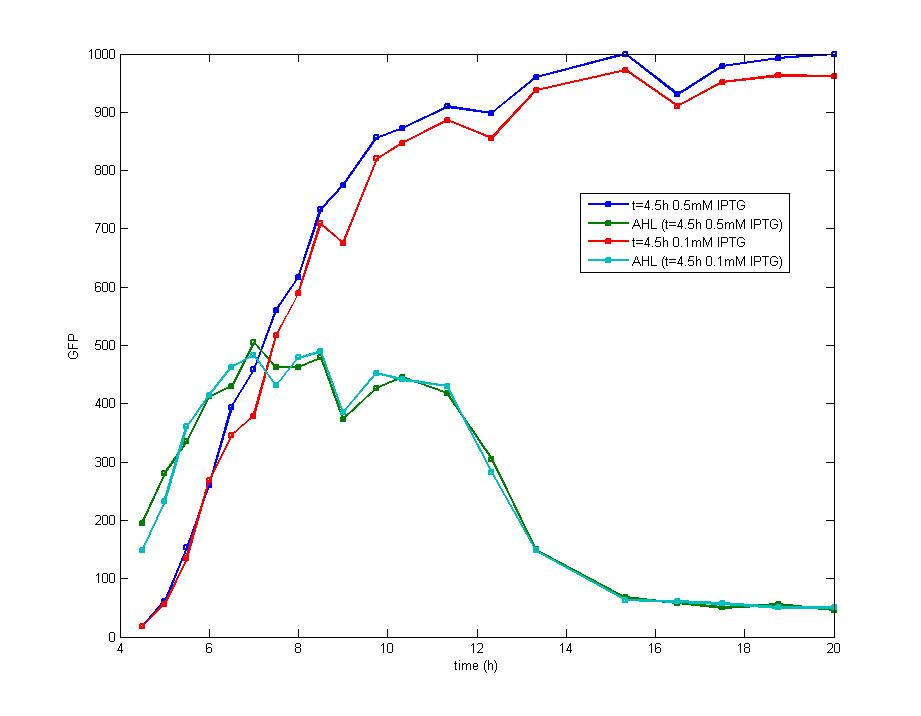
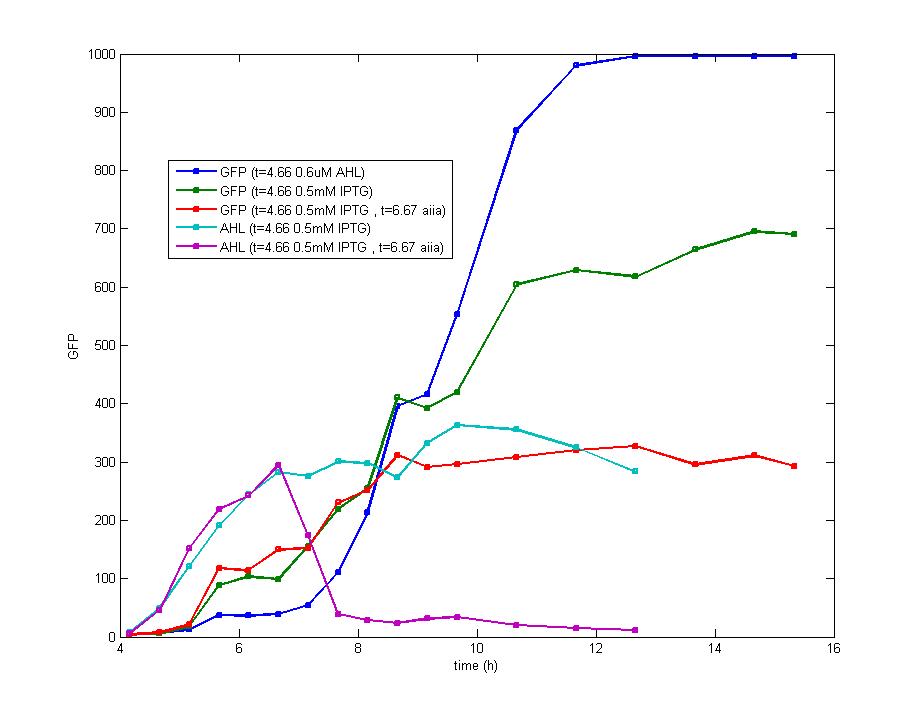
| - | Figure 5. The addition of IPTG with a concentration of 1 mM and 0.1 mM individually at the inoculation makes no diversity on the induction behavior ,but the fluorescence intensity varies at the highest level. <br> | + | Figure 5. The addition of IPTG with a concentration of 1 mM and 0.1 mM individually at the inoculation makes no diversity on the induction behavior ,but the fluorescence intensity varies at the highest level. <br><br> |
| - | <br> | + | |
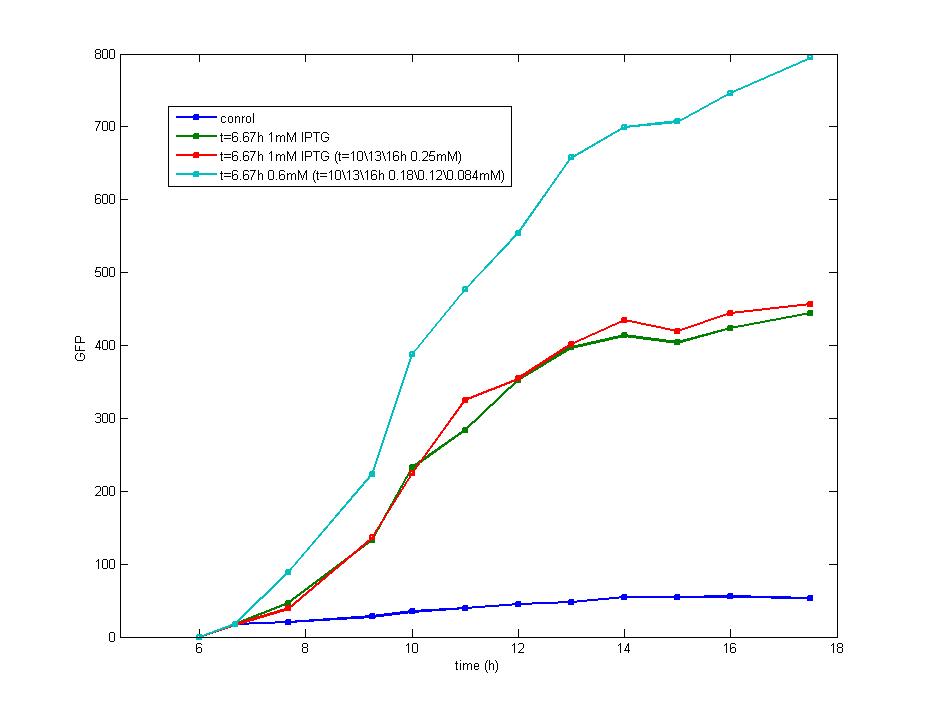
Based on the above analysis, we conclude:<br> | Based on the above analysis, we conclude:<br> | ||
| - | + | a. The early exponential phase is most suitable for the addition of IPTG which could repress the expression of luxR effectively and provides more data before cells enter the stationary phase. The optimal bacteria density for the addition of IPTG is the range of 0.2(OD<sub>600</sub>) to 0.3(OD<sub>600</sub>).<br> | |
b. The minimum concentration of IPTG required to induce the lac promoter is very low and has no direct influence on the final result,]. Besides, the induction of IPTG is so focused on the beginning that it could be treated as an instant signal, it is unnecessary to add more IPTG after the first addition. The optimal concentration of IPTG for induction is 0.5mM. | b. The minimum concentration of IPTG required to induce the lac promoter is very low and has no direct influence on the final result,]. Besides, the induction of IPTG is so focused on the beginning that it could be treated as an instant signal, it is unnecessary to add more IPTG after the first addition. The optimal concentration of IPTG for induction is 0.5mM. | ||
<br><br> | <br><br> | ||
Revision as of 19:45, 26 October 2007
[http://www.example.com link title]