Caltech/Project/Constructs
From 2007.igem.org
| Line 10: | Line 10: | ||
|style="background:#ffffff"| | |style="background:#ffffff"| | ||
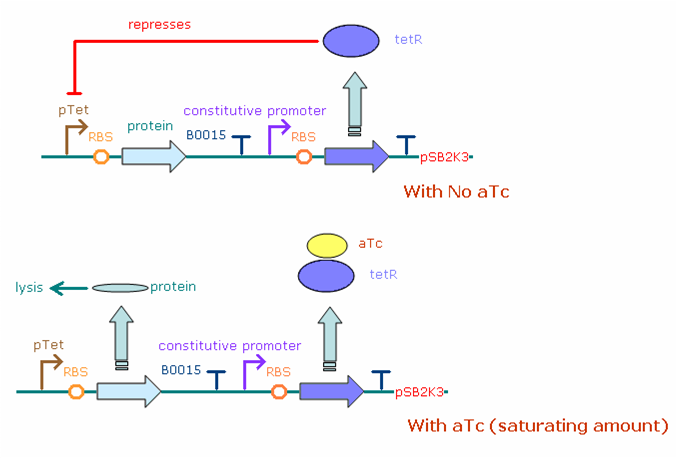
| - | The figure below illustrates the | + | The figure below illustrates the expected behavior of the final construct system in ''E. coli'' bacteria. A constitutive promoter (both J23100 and J23116) continuously produces transcripts of the tet repressor gene, which when translated turns into tet repressor protein (tetR). The pTet promoter drives expression of the desired protein, N/Q/cro or YFP. In the absence of aTc, the tetR binds to pTet, greatly reducing the number of protein transcripts (and thus the protein concentration) in the cell. Adding aTc increases the protein concentration, as aTc binds to tetR and frees the pTet promoter to produce more transcripts. The pTet system allows us to reach intermediate expression levels, rather than switching between 'on' and 'off' states. |
| - | [[Image:constructExplanation.png | + | [[Image:constructExplanation.png|600 px|center|frame|<center>Construct Behavior: With no aTc in the system, tetR represses pTet promoter activity, resulting in a small number of the “protein” in the cell. With the addition of a saturating concentration of aTc, all the tetR protein is bound, leaving the pTet promoter free to produce the viral proteins that will lead viruses towards the lytic pathway.</center>]] |
| - | <center>Construct Behavior: With no aTc in the system, tetR represses pTet promoter activity, resulting in a small number of the “protein” in the cell. With the addition of a saturating concentration of aTc, all the tetR protein is bound, leaving the pTet promoter free to produce the viral proteins that will lead viruses towards the lytic pathway.</center> | + | |
| - | |||
| - | As the cis and trans riboregulators had not yet been designed when the cloning process started, a system with tetR(with parallels to ''cis'' repression) and aTc(parallels to ''trans'' activation) regulation of protein production was chosen instead, so that verification of lysis could begin on the viral protein constructs. As the recombineered amber phage have not yet been completed, constructs were made in bacterial (D1210) plasmids (pSB2K3). The D1210 strain was chosen for its extremely low-copy number, as was the plasmid (only inducible with IPTG), | + | As the cis and trans riboregulators had not yet been designed when the cloning process started, a system with tetR (with parallels to ''cis'' repression) and aTc (parallels to ''trans'' activation) regulation of protein production was chosen instead, so that verification of lysis could begin on the viral protein constructs. As the recombineered amber phage have not yet been completed, constructs were made in bacterial (D1210) plasmids (pSB2K3). The D1210 strain was chosen for its extremely low-copy number, as was the plasmid (only inducible with IPTG), so hopefully has only one copy of the current plasmid. This combination therefore further simulated future infection conditions, where only one phage would infect each cell. |
|} | |} | ||
</div> | </div> | ||
Latest revision as of 23:29, 26 October 2007
iGEM 2007
Home
Highlights
Project
People
Protocols