Arthur Yu Notebook
From 2007.igem.org
My Construction Files
My Sequencing Files
6/15 digestion party
- Good D1, D4
- synthy plasmid thingy...
# [digest] kristin B4 for backbone. Used EcoRI/XhoI purified L # [digest] synthy plasmid thingy for insert. Used EcoRI/XhoI purified S
- I716003a (pBca9145- cmr cass+rbs+neuS)
# [digest] pBca9145-neuS (I716001) (BglII, XhoI; 2063+1245; S) # [digest] pBca1101-I716051 (BamHI, XhoI; 3119+ 850; L)
- pBca9145-yfbE_pro-rbs-RFP (I716004)
# [digest] pBca9145-yfbE_promote (I716002) (EcoRI/BamHI, 2063+ 421, S) # [digest] pBca1100-Bca1109 (EcoRI/BamHI, 2927+1253, L)
- wbbL
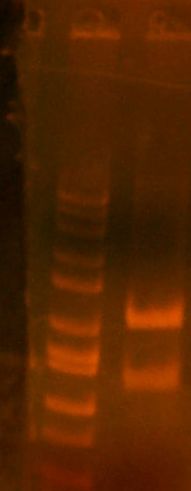
# miniprep'd and ready to go! # [IMAGE] of gel # Sent E1 and E2 for sequencing, forward (ay014, ay015)
NOtes: STILL NEED TO ENTER YFBE PROMOTER PART LOL entering composite parts would be nice too
I did 10 digestions today. I'm proud of myself.
6/14 stuff about things
- neuS clone C1: WE HAVE A WINNER!
- miniprep party, D1/2/3/4
- digestions didn't work too well mmmm going to sequence D1 and D4.
- wbbL good plate, now incubating in shaker
- cgctattcgcgctacctttg ready to order (middle sequencing of HPI/katG)
6/13 gloves, zymo, and ethanol oh my
- a random day
- neuS digest used to transform n plate new colonies, since the old plate had only 3 people, and 1 which worked
- wbbL digest > new plate as well (old one had one colony and it was bad)
- yfbE put into shakey tubes
- One of the neuS got miniprepped and the test digest looks good compared to test in ApE
- sending it for sequencing, eh.
- Sequencing...
- Most (A1, A4, B1) sucked
- only A3 (HPI/katG) was decent. It might have an addition mutation of a G.
- A3 sent for reverse sequencing with G01001
- Began redo-ing of HPI/katG-making, with a phase 1 PCR (the halves with a mutation)
Todo: Input parts in registry (yfbE?)
6/12 a bag full of grapes
- YAY WE ALL GOT OUR OWN SET OF PIPETTE PEOPLE
- PCR of yfbE...
- last night's thing, left in the freezer overnight. >FAIL<
- Did a new PCR -- looks good -- cells xform'd, plate is incubating.
- neuS new xformation looks good. Three colonies now incubating.
- wbbL (1) and HPI/katG (4)
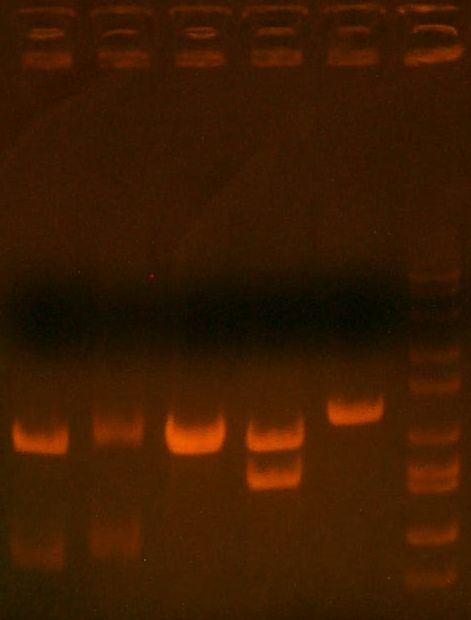
- miniprepped and digest gel ran:
- HPI/katG 1,2,3,4 || wbbL || marker
- 1,2 might be okay.. that faint band is weird. 3 is great! 4 = wtf. wbbL = wtf too (should have two bands)
- decision to put 1,3,4,wbbL for sequencing.
Ayu 17:59, 12 June 2007 (EDT)
6/11 austin's birthday
- CAKE PARTY - great custard cake
- I put the wbbL (1) and HPI/katG (4) colonies to incubate in LB broth.
- neuS failed; no colonies :(((((((
- redid ligation and xformation. hopefully there will be good results tmrw!
- made like 20 LB-Agar/Amp plates - looks like our stock will last at least this week
- researched nitric oxide (NO) and E. Coli - looks like soxRS is promising
- also researched RBCs and how they deal with NO
- plopped yfbE into PCR will do stuff with it tmrw
TO DO: enter yfbE into the registry
Ayu 18:24, 11 June 2007 (EDT)
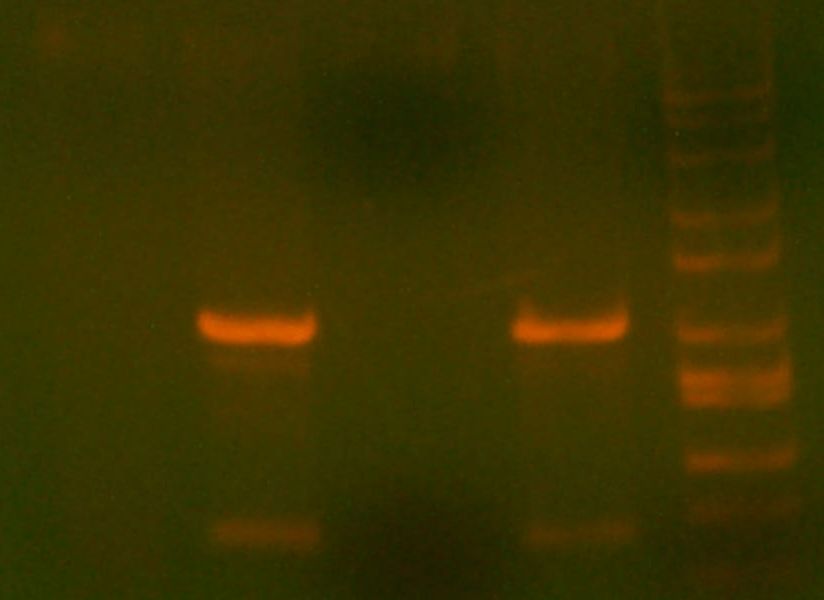
6/8 long day?
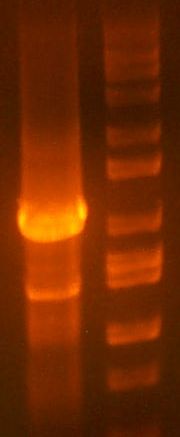
- My PCR from last night (HPI/katG) was ROXOR! (left)
- xformed some DH10B's. w00t w00t
- Today's PCR was wbbL and neuS. ALSO ROXOR LOL (right)
- xformed DH10B's.
- made oligos for yfbE promoter thingy - will test with GFP and yeah! next week!
- poured lotsa LB/agar+amp plates
6/7 we got benches
- we got benches
- pcr of [http://partsregistry.org/Part:BBa_I716253:Design HPI/katG from Salmonella]
- well... getting the mutated PCR prod overnight. going to xform tmrw, hope it works!
- programmed pcr on machine upstairs (#6)
- we got computers
- AGAR SUX, for future reference:
- nuke @ 20:00 min, 50% power.
- water bath in tap water for 5-10 min
- thaw the antibiotic right now!!
- FIRE for disinfecting
- pour that stuff. set 15 min, then marker it then bag
6/6 waiting for oligos
- Made oligos and constructs with Vai, for getting wbbL and neuS from pJ23006-Bca9106
- We tried the P_tet/RFP triple/double digest to make a composite part.
- FAIL
- probably source DNA is bad
- so much for that activity...
Other stuff: I won speed scrabble. even though I kind of cheated ish (didn't stop when Sam said stop"
6/5 coolbeans
- Finalized oligos to order with Vai
- Learned about LB broth-ing and LB/Agar plating. Thanks, Austin and Sam :)
- Learned about the many composite part-making methods. Props 2 Chris
- prefix/suffix is weaksauce
- Use the AlwnI or BsaI or BglI, in conjuction with BglII or BamHI << (Did this today)
- DBBS
- 3 antibiotic; MIT endorses, used for BioBrick 1.0. Triple digest = bad
- 1-2-3 method << 'Our Goal' in a few weeks. should be leet.
- Planned and vicariously did the making of P_tet+RFP brick (see Vai Notebook)
Other Notes: All oligos are being ordered, w00t w00t.
Ayu 18:36, 5 June 2007 (EDT)
6/4 Training Finishes, Real Stuff Starts
- Incubated some colonies
- Miniprep'd already-been-incubated colonies (2)
- Double digest of the 2 minipreps + parent plasmid
- Colony PCR'd the incubated E.coli
- Ran gel of the digest + PCR
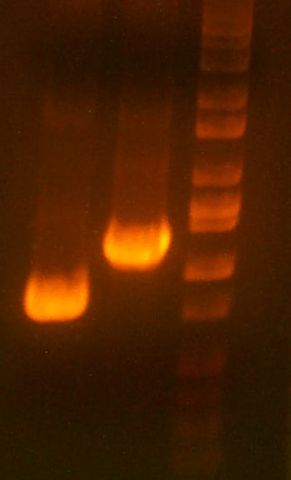
- >>> PCR product / Miniprep 1 / Parent Plasmid / Miniprep 2 / Ladder >>>
- No bands for PCR or parent. Confused? Other ones look great.
As for me: Wiki acc works now.
Designing oligos and will compare with Vai.
Ayu 18:19, 4 June 2007 (EDT)
to do