Duke/Projects/efatf
From 2007.igem.org
Contents |
Electric field-activated transcription factor
Background and Motivation
Experience in previous iGEM contests has shown that genetic circuits can be fickle, noisy and inhomogeneous, in contrast to electric circuits which are easily controllable but lack the breadth and variety of biological components. In order to enhance the controlability of genetic circuits, we are targeting the lifeblood of genetic circuits, the transcription factors, by attempting to guide their function with macroscopic inputs. The goal of this project is the creation of an electric-field activated transcription factor protein which will initialize transcription only in the presence of an applied, low-strength (10^3-10^5 V/m) alternating electric field.
The design aims to accomplish this goal by modifying the ‘flexible arm’ of the λcI repressor such that an applied electric field will stabilize the altered protein in an otherwise unfavorable binding with its operator site. λcI repressor This can be accomplished by screening a library of 'flexible arm' mutants, the results of which can be further screened using directed evolution techniques to yield the protein which matches the above specifications. As most proteins are fairly resistant to conformational perturbations due to electric fields , this project focuses on isolating those amino acid sequences which are particularly susceptible to electric fields.
Parts and setup
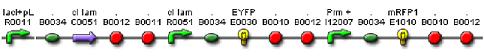
We constructed a genetic circuit as an apparatus to test whether the mutant λcI repressors would bind to DNA or not under a variety of conditions. We combined [http://partsregistry.org/Part:BBa_I5010 BBa_I5010] and [http://partsregistry.org/Part:BBa_I6036 BBa_I6036] with [http://partsregistry.org/Part:BBa_I12007 BBa_I12007] and [http://partsregistry.org/Part:BBa_I13507 BBa_I13507] to yield [http://partsregistry.org/Part:BBa_S03819 BBa_S03819], shown below.
BBa_S03819 contains the λcI promotor (I12007) and repressor (R0051) sequences, nearly identical parts derived from phage λ, which give the λcI repressor protein the ability to activate or repress transcription depending on where the two sequences are placed in relation to the target protein. Thus, BBa_S03819 turns bright RED when the λcI repressor is able bound to DNA (can initiate transcription at I12007 and represses transcription at R0051) and glows YELLOW when λcI repressor is unbound (does not repress transcription at R0051 and does not activate transcription at I12007).
BBa_S03819 was then PCR'd with primers 5’-ccttggatccatAAAGAGGAGAAATACTAGATGNNNNNNNNNAAGAAACCATTAACACAAGAGCAG-3’ or 5’-ccttggatccatAAAGAGGAGAAATACTAGATGNNNNNNNNNNNNNNNCCATTAACACAAGAGCAG-3’, primers with a BamHI restriction sites with random nucleic acid N sections to yield mutants of either the first three amino acids of the flexible arm or the first five amino acids of the flexible arm, respectively, with 5’-aaggggatccgtgctcagtatcttgttatcc-3’; the PCR product was then a linear library of mutant BBa_S03819. The three amino acid mutants (called in our lab 'mut3') and the five amino acid mutants ('mut5') were then digested with BamHI, ligated, transformed, and screened for DNA binding under a variety of conditions.