Taipei
From 2007.igem.org
Title Regulated insulin secretion system in E.coli
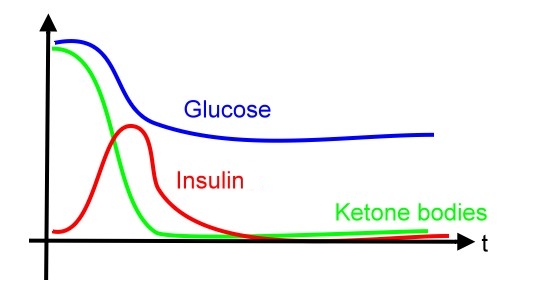
Motivation Over 7 percent of adults in the United States (US) are known to have diabetes mellitus. However, because of the associated microvascular and macrovascular disease, diabetes accounts for almost 14 percent of US health care expenditures, at least one-half of which are related to complications such as myocardial infarction, stroke, end-stage renal disease, retinopathy, and foot ulcers. Compared to well-known type 2 diabetes mellitus, manifested with different degrees of insulin resistance, type 1 diabetes mellitus, one of the most common chronic diseases in childhood, is caused by insulin deficiency following destruction of the insulin-producing pancreatic beta cells. Data from the Center of Disease Control (CDC) show that more than 13,000 children and adolescents (19 years of age) are diagnosed with type 1 disease each year with a prevalence of 1.7 per 1000. Control of blood sugar and prevention of severe complications, such;as diabetic ketoacidosis (DKA), are important issues nowadays. Diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state (HHS, also known as nonketotic hyperglycemia) are two of the most serious acute complications of diabetes. They are part of the spectrum of hyperglycemia and each represents an extreme in the spectrum. DKA is characterized by the triad of hyperglycemia, anion gap metabolic acidosis, and ketonemia. Metabolic acidosis is often the major finding. The serum glucose concentration is usually greater than 500 mg/dL (27.8 mmol/L) and less than 800 mg/dL (44.4 mmol/L). Three ketone bodies are produced in DKA: two ketoacids (beta-hydroxybutyric acid and acetoacetic acid), and one neutral ketone (acetone). The treatment of DKA and HHS is similar, including the administration of insulin and correction of the fluid and electrolyte abnormalities that may be present, including hyperglycemia and hyperosmolality, hypovolemia, metabolic acidosis (in DKA), and potassium depletion. Existed works (see References below) are focusing on how to take advantages from properties of mammalian cells to express and secrete insulin in a trail-and-error manner. However, from the perspective of synthetic biology, it is possible to engineer a cell works as we want, no matter what type of cells we apply to.
Goal and system design
For the iGEM 2007 project, our team focuses on the development of a prokaryotic system to
express insulin regulated by external glucose concentration and
clean the ketone bodies accumulated by mamalian cells due to insufficient intake of glucose
balance between glucose and insulin to stablize glucose level and prevent low blood glucose condition
system specficiation is shown below:
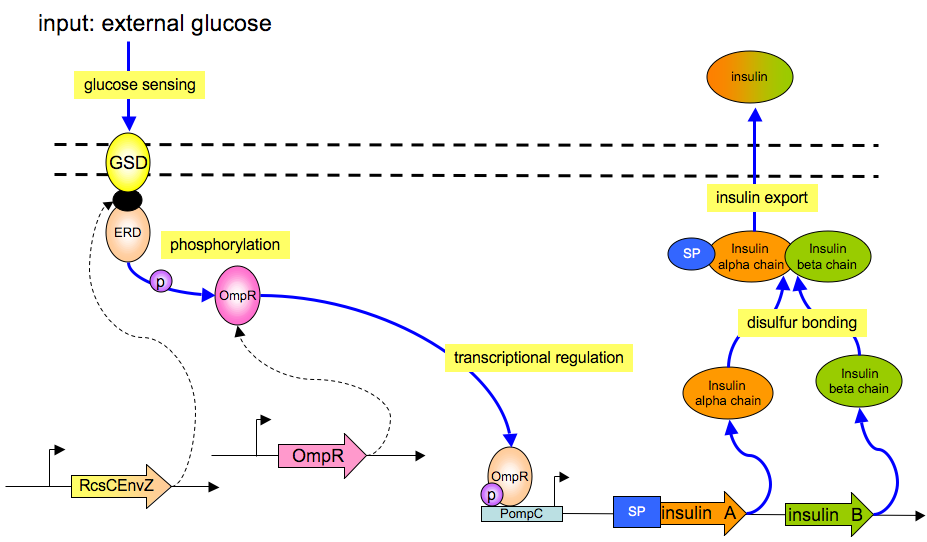
The basic architecture for our insulin secretion design is shown in the figure below:
External glucose can be sensed by GSD (Glucose Sensing Domain) from gene RcsC and transmits response to ERD (Responsive Domain from EnvZ gene)
TCS phosphorylation relay activates the expression of insulin (both alpha and beta chains)
With signal peptide (SP), the assembled insulin (by disulfide bonding) can be exported outside the membrane of the prokaryotic cells
Implementation part list circuit design lab notes
References Engineered enteroendocrine cells secrete insulin in response to glucose and reverse hyperglycemia in diabetic mice, 2007 Mol Ther. Development of genetically engineered human intestinal cells for regulated insulin secretion using rAAV-mediated gene transfer, 2003 Biochem Biophys Res Commun http://www.utdol.com/utd/content/topic.do Recombinant DNA Technology in the Synthesis of Human Insulin Glucose-Dependent Insulin Release from Genetically Engineered K Cells, 2000 science [http://www.nature.com/gt/journal/v7/n21/abs/3301306a.html Glucose-stimulated and self-limiting insulin production by glucose 6-phosphatase promoter driven insulin expression in hepatoma cells, 2000 Gene Therapy]
Please visit our external site [http://igem.ym.edu.tw http://igem.ym.edu.tw] and [http://igem.ym.edu.tw/iGEM2007/index.php/IGEM2007_project our project]