Toronto
From 2007.igem.org
Link to our website: [http://www.igem.skule.ca BlueGenes Website]
Contents |
Project: E. Coli Neural Network
INTRODUCTION
Neural networks (NNs) are nonlinear systems capable of distributed processing over a number of simple interconnected units. By adjusting connections between these units, NNs are capable of learning. Similar to NNs, a culture of Escherichia coli cells can be viewed as a distributed processing system with connections formed by intercellular signaling pathways. By engineering the cells to interact with each other in predetermined ways, it should be feasible to develop a bacteria based neural network.
OBJECTIVES
The goal of the proposed project is to develop a simple two-unit feedforward NN using E. coli cells. The network will consist of one input unit and one output unit. This network will be trained to either allow the input to propagate to the output, or to block propagation.
REVIEW OF PERTINENT LITERATURE
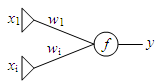
The neural network topology being examined in this proposal is known as a perceptron. Perceptrons are linear classifiers, which output the function of a linear combination of inputs [1]. The factor by which each input is multiplied is known as the weight. A perceptron is visually depicted as a layer of input units which are connected by network weights to a single output unit which performs a function on the weighted sum of the inputs (Fig. 1). For the proposed project, only one input (x1) will be used.
Training of feedforward neural networks typically employs a method known as backpropagation [2]. Backpropagation involves a forward pass where the output of the neural network is computed for a given set of inputs. A backward pass is made where network weights are adjusted based on the error between the actual output and the expected output. The delta rule is commonly employed to determine the amount of weight change. For perceptrons, a simplification of the delta rule can be made where the weight change is equal to (delta)wi = a xi (d-y) (1).
where d is the target output and alpha is a constant that controls the amplitude of the weight adjustment [1]. Training is done by exposing the network to a training set until the error between the actual and expected output decreases within a defined tolerance level.
Fig. 1. Generalized topology of a perceptron.
METHODS
A. Basic E. Coli Neural Network Topology
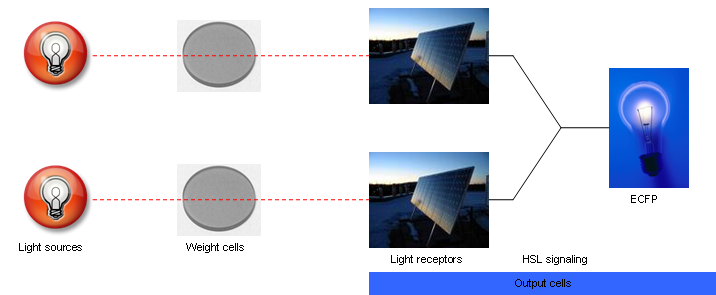
Rather than using single cells to represent individual NN units, the proposed design treats groups of cells as individual units. These cells will either be in an active or inactive state. Using this approach, network connection weights can be represented by the concentration of active cells containing a particular plasmid vector. A larger concentration of active cells with a particular plasmid would result in a faster net production rate of output signal molecules per unit area (i.e. a stronger weight). Thus, for a two unit NN, two different vectors would be needed – one for the input unit and one for the output unit. It may be possible, however, to combine these two vectors into a single vector for the sake of simplicity.
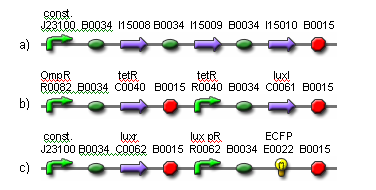
The vector for the input layer cells is shown in Fig. 2a-b. These cells can be in an active or inactive state, which is established by a toggle switch consisting of two inverters (cI lam and tetR). When active, they produce an intercellular HSL signaling molecule (3OC6HSL) in response to red light (660 nm) via expression of luxI. When the cells are inactive, this production is suppressed.
The vector for the output layer cells is shown in Fig. 2c. The output layer cells constitutively express luxr which detects 3OC6HSL. In response to the HSL from the input unit cells, the output layer cells produce a short lasting ECFP. These features provide the basic NN functionality.
Fig. 2. Basic neural network functionality components. (a) The input cell component consists of a bistable toggle switch that controls cell activation state. (b) When the cell is active, the absence of tetR allows the transcription of light sensing proteins. When light is detected, 3OC6HSL is produced via luxI transcription. (c) The output cell component detects 3OC6HSL via luxr. This in turn activates transcription of ECFP.
B. Backpropagation Controller
Backpropagation is performed by adjusting the network weights. This is done by deactivating input cells to decrease their weights or activating them to increase their weights. The training mode is activated in the presence of arabinose. While the network is in training mode, heat provides the training signal. In order to enable this network to learn via backpropagation, further functionality is added to the input layer cells to allow activation via O3C14HSL and deactivation via AI-1. A backpropagation controller cell type is also introduced.
Heat and 3OC6HSL from the input layer only need to be detected in the presence of the input signal (light) due to the x term in (1). Light sensitive proteins are produced in when training mode is initiated via arabinose (Fig. 3c). To detect heat, expression of temperature sensitive lacI is enabled in the presence of light (Fig. 3d). When heat is detected, the backpropagation cells produce O3C14HSL via cinI (Fig. 3d). In the presence of light, 3OC6HSL from the input layer results in the transcription of lasI and thereby the production of AI-1(Fig. 3e).
O3C14HSL and AI-1 modulate the activation state of input layer cells. O3C14HSL promotes activation of the input unit cells via cI lam (Fig. 3a), while AI-1 promotes deactivation via tetR (Fig. 3b). In essence, these two intercellular signals compete to activate or deactivate the input layer cells, providing the d-y term in (1). The duration of training provides the α term.
Fig. 3. (a) Detects O3C14HSL using cinR and promotes activation of the input cells via cI lam. (b) Detects AI-1 using lasR and promotes deactivation of the input cells via tetR. (c) Produces heat and light sensing proteins for the output cells when training mode is activated by arabinose. (d) Produces O3C14HSL via cinI when heat is detected in training mode. (e) Produces AI-1 via lasI when light is detected in training mode.
Training
This network will be trained to either allow or block propagation of the input to the output.
1) Allow propagation of the input to the output: This will involve providing an input and training signal while the network is in training mode.
2) Block propagation of the input to the output: This will involve providing an input signal without a training signal while the network is in training mode.
Ideally, the cells will be in solution to allow random movement, thereby allowing for more active competition between the O3C14HSL and AI-1 signals.
CONTINGENCY
The most complex modules are the ones that provide backpropagation functionality. If these modules fail, one could develop a vector that outputs O3C14HSL or AI-1 in response to manual input signals and manually provide the weight adjustment signals.
If one of the HSL components does not work, it may be possible to feed the input cell output signal back into the input cells, thereby reducing the need for one of the HSL components. This is not optimal as we are interested in what the output cells detect rather than what is detected by the input cells. As such, doing this may require more tweaking of the cell density to ensure a fairly uniform concentration of HSL molecules.
It should be noted that the constitutive expression promoters can be changed to modulate the expression level of the HSL sender and receiver components.
SIMULATION
Simulation will need to model the internal chemistry of the cell, as well as the propagation of intercellular signals outside the cell. This will help determine wait times required for an input signal to propagate to the output and for network training take place. The model will also help form an idea of expected background noise.
The modeling process will first involve constructing a model of the basic neural network functionality. Once this is working as tested by hypothetical inputs, the backpropagation components can be added. The following provides a guideline on some of the constants that need to be determined to model the basic system.
1) Toggle switch: transcription, translation, promoter binding and degradation rate constants of tetR and cI lam.
2) Light sensor: transcription, translation, degradation and promoter binding rates of the light sensing proteins, the transcription and degradation rates of luxI and the rate at which luxI produces 3OC6HSL.
3) Output layer: Once the intracellular concentration of 3OC6HSL is modeled, the binding and reverse binding constants of the HSL to the cell’s surface will need to be determined. For simplicity, it may be assumed that there are a fixed number of receptors on the cell’s surface. A model will need to be proposed for the method of signal transduction that results in the transcription of ECFP. The transcription, translation and degradation rates of ECFP will also need to be measured.
SIGNIFICANCE OF WORK
Developing a NN using a genetic regulatory network would provide a stepping stone for the creation of biological systems that operate on fuzzy logic. These types of systems would have the ability to adapt to changing environmental conditions without the need to design traditional predicate logic based genetic circuitry.
While relatively simple in functionality, this design will provide a basis for more complicated NN topologies. For example, with a little more work, one could expand the input layer to contain two input units. This network topology would then be capable of being trained to behave as an AND gate or an OR gate. A constant input and the capacity for negative weight values could also be implemented to allow for situations where there is output without input, as is the case for NAND and NOR gates.
REFERENCES
[1] O. Weisman, The Perceptron. [Online]. Available: http://www.cs.bgu.ac.il/~omri/Perceptron
[2] B. Bardakjian, Cellular Bioelectricity Course Notes. 2005.
Team Members
Groups within the team, and current contact people
- Finance (Kirill, Conrad)
- Design (Natalie, Charles)
- four projects under feasibility evaluation
- Lab (Charles, Natalie)
- core: 4
- rotation: 12+ confirmed
- Presentation (Andy)
iGEM 2006 Wiki: [http://parts2.mit.edu/wiki/index.php/University_of_Toronto_2006 Blue Water]. Of particular note is the lab notebook (click on Construction under Committees).
Editors of this wiki: Markup conventions can be found on the [http://en.wikipedia.org/wiki/Wikipedia:How_to_edit_a_page Wikipedia editing tips].
Much more to come.