Turkey/ Possible Projects
From 2007.igem.org
Project 1 Mexican Wave
FIRST VIEW
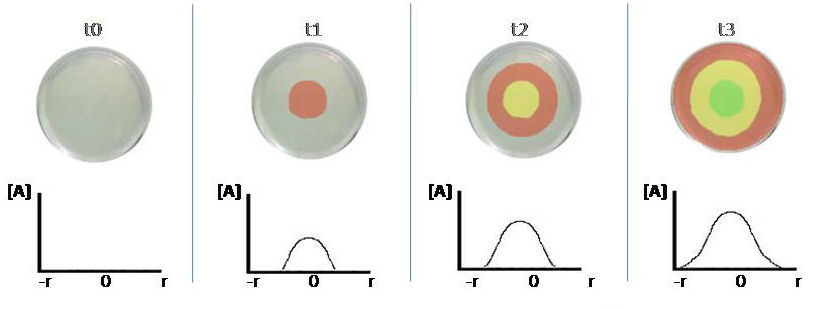
- Our first thought was to create a pattern by the change of colors in a concentration dependent manner; so that the production of a protein at the end of a different event would trigger the pattern formation, just like a goal triggering a *Mexican wave in soccer games.
- The plan was to have a plate having a lawn of same type E. coli cells having 3 constructs. Each construct would have a promoter activated by a different level of the initial signal protein and a reporter fluorescent protein coding part.
- The triggering protein A is being dropped on the middle of the plate, creating a concentration gradient on the plate as in the figure. Acording to this plan the color change will be once, and then by the rising level of [A], the plate will all become green.
SECOND VIEW
- The second view was to make this color change continuous and independent of a created concentration gradient. So first idea came up to be coupling this oscillation to an already oscillating protein, maybe to cell cycle. But then this oscillation will be observable only on a single cell unless we synchronize the cell cycles of different cells on the plate.
Project 2 Chase Simulation
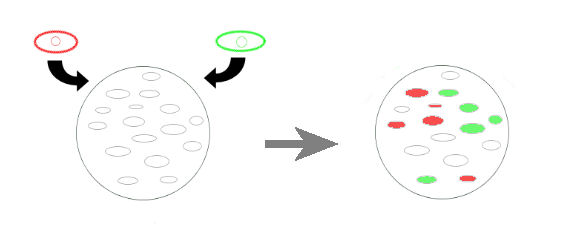
- The first idea of project was to simulate the competition between two different warrior cell types to invade a plate having a lawn of passive cells. The green and red cells are the invaders, and they represent two teams. The 'empty cells' are the ones to be invaded. Empty cells have two constructs, they are normally off and each construct can be activated by a red or green cells, invaders. If one of the constructs in empty cells is activated, this construct irreversibly closes the other constructs.
- The trick point in this project is to have two promoters which are normally off, and also repressed and activated by distinct proteins. There should be no leakage, because a little leakege in one of the constructs closes the other irreversibly. So we created a less complex version;
- We reduced the invaders to one type, and each team will have a separate plate. This invader will activate a normally off promoter which codes for a flourescent protein. In the registry there are there pairs of promoter-activator for this purpose. But the experience info is inadequate, so we will try all of them. This way we can overcome the leakage problem, if there is a constitutive leakege, there will be stable expression of the protein unless the invader is on action. So we can make a base level correction to see the effective contrubution of invasion.
- The Three pairs of promoter-activator are:
PartPartInfoPlateWellPlasmidLengthCellSel. BBa_B0015Double terminator11IpSB1AK33189 3318 445V1001AK BBa_B0015Double terminator33OpSB1AK33189 3318 445V1010AK BBa_b0034RBS13OpSB1A22079 2091 250V1004A BBa_I14032constitutive promoter217KpSB2K34425 4462 353V1006K BBa_E0020E-CFP17CpSB1A22079 2802 961V1001A BBa_E0030E-YFP17GpSB1AK33189 3912 1039V1003AK BBa_E0044GFP15BpSB1A32157 2916 1075V1001A BBa_J06504mRFP27NpSB1A22079 2793 952V1009A BBa_I12007lambda Prm promoter activated by cI215KpSB2K34425 4507 398V1006K BBa_C0051cI repressor from E. coli phage lambda (+LVA)15GpSB1A22079 2829 988V1004A BBa_R0062promoter activated by HSL&LuxR19GpSB1A22079 2134 293V1004A BBa_C0062luxR repressor/activator17ApSB1A22079 2835 994V1004A BBa_C0061Synthesizes 3OC6HSL, which binds to LuxR15OpSB1A22079 2697 856V1004A BBa_R0079Promoter (LasR & PAI regulated)119NpSB1A22079 2236 395V1001A BBa_C0179LasR activator13JpSB1A22079 2802 961V1003A BBa_C0078autoinducer synthetase for PAI27IpSB2K34425 5067 958V1004K BBa_R0080Promoter (AraC regulated) 121LpSB1A22079 2228 387V1001A BBa_C0080araC arabinose operon regulatory protein27MpSB2K34425 5340 1231V1004K BBa_I1051Lux cassette right promoter 118PpSB1A22079 2147 306-A