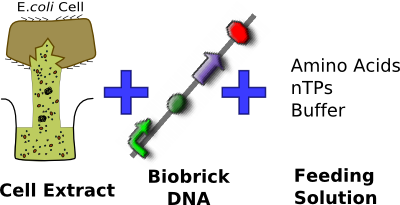Imperial/Infector Detector/Design
From 2007.igem.org

Infector Detector: Design
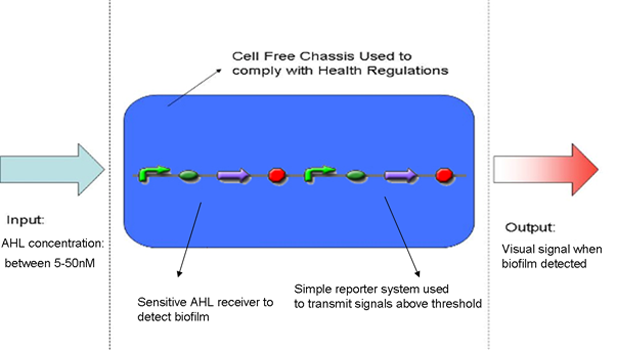
Design Overview
| Property | Value | Design Solution | System Level |
| Health Regulations | System Must not be living replicating bacteria | Use a Cell Free System e.g. Promega's S30 Cell Extract | Chassis |
| Lifespan | System must have a shelf life of 7 days | Protease Inhibitor of Cell Extract should ensure degradation of Visual Reporter is Minimal Proper Packaging should ensure that evaporation of Cell Free system is so low that system can surive for 7 days | Chassis |
| Inputs | System must be sensitive to AHL concentration between 5-50nM | Sensitive AHL receiver to detect low AHL levels | Construct |
| Outputs | System must give a visual signal if bacteria is present | Couple AHL to a reporter system expressing fluoresent protein eg. RFP | Construct |
| Operating Conditions | System must operate within temperature 20-30°C | Gene expression systems and protein must be thermostable | Construct |
| System must operate within pH range of 6-8 | Gene expression systems and protein must be pH resistant | Construct | |
| Response Time | System needs to have a response time under 1 hour | To be Determined - this is hard to design for | Both |
DNA Constructs
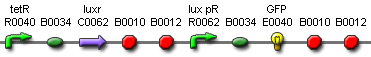
Our first aim is to create a system that can accurately detect the concentration of AHL in solution. We needed a well characterized detector system sensitive to 3OC6HSL which we will attach to a standard GFP reporter to determine its response to GFP. The first construct is appealing as the AHL receiver in front is well-documented as part [http://partsregistry.org/Part:BBa_F2620 BBa_F2620], thus giving more assurance in the reproducible nature of our results.
However, the weakness of construct 1 lies in the fact that it does not respond uniformly to constant AHL concentration since the activator protein LuxR is produced by a promoter and not maintained at a constant level. This makes it hard to relate the output of the system to the AHL input. We thought that if purified LuxR could be controlled and added to the system instead (simplify the construct by removing the constitutive LuxR production), this would give us more controllability on the input, thus better reproducibility.
Chassis Selection
We have chosen to use the commercially available S30 E. coli cell extract made by Promega. After having looking into a variety of different cell-free chassis, we feel that this chassis best suits our needs. In particular this chassis allows us to meet our base requirement of complying with the Health and Safety regulations of the field we are working in, as we do not want replicative bacteria that could potentially be pathogenic to come in contact with urinary catheters.
In addition to complying with health regulations the S30 cell extract is commercially available meaning that it has been shown to work. This is very important for us as it allows our focus to be on tuning the chassis to suit our needs rather than making the chassis work in the first place.