Saint Petersburg
From 2007.igem.org
Contents |
Saint-Petersburg iGem2007 team
This year our team is participating for the first time in iGem2007 project.
Our team works at the [http://www.spbstu.ru Saint-Petersburg State Polytechnical University] in the department of Biophysics.
About Us
- Students:
- Our Instructors:
- Vasiliy Romanov
- Alexej Skvortsov
- Our Sponsors
- [http://www.dynastyfdn.com Dynasty Foundation]
- [http://www.spbstu.ru/english/phmech/phmech_about.html Faculty of physics and Mechanics], St-Petersburg State Polytechnical University
- [http://www.spbstu.ru/english/coordination.html Research & Development Complex] of St-Petersburg State Polytechnical University
The members of the team are thankful to the sponsors for the possibility to take part in the IGEM project.
Our IGEM-2007 Project
Introduction: Copper and Copper Toxicity
[http://en.wikipedia.org/wiki/Copper Copper] is a unique transition element, that may easily activate dioxygen bond. This makes copper both vitally essential to aerobic species and severely toxic to all organisms at higher concentrations. The toxicity is caused mostly by catalyzing uncontrolled dioxygen conversion to reactive oxygen species via a series of redox reactions (electrochemically similar to [http://en.wikipedia.org/wiki/Fenton%27s_reagent Fenton cycle]). To avoid this problem most organisms evolved copper metabolic systems, so that virtually all copper inside the cell is tightly protein-bound. Still high external concentrations of copper is deadly to many organisms, underlying the well known antifungal and insecticide applications of copper salts in agriculture. Yet copper is not so evident toxin as nickel or cadmium, as many organisms (e.g. higher plants) are resistant to high concentrations of copper.
In our opinion, importance of copper as water pollutant and ecotoxicant is underestimated, probably because its toxic action on natural ecosystems is not so evident.
For humans the main source of excess copper ions is drinking water. The potential copper ion sources are agricultural application, metallic copper corrosion and chemical industry. Adult mammals have a protective system, that controls copper absorption in the intestine and copper excretion through bile. E.g., for adult rats, LD50 oral dose of Cu salts is over 30-fold higher than intraperitoneal LD50 dose (580 vs 15 mg/kg, [http://ptcl.chem.ox.ac.uk/MSDS/CO/copper_II_chloride.html Safety data for copper (II) chloride]). But newborn infants have other type of copper metabolism. Copper absorption and excretion in suckling newborns is not controlled. Copper is provided in exact amounts in protein bound form by milk. So the infant totally relies on delicate copper control of the mammary gland of its mother. This scheme leads to the existence some very peculiar copper disorders, like “toxic milk mice”. The fact means, that copper is a very dangerous element for infants. Excess copper, absorbed by an infant, may inflict serious disorders on the developing brain and cause many delayed problems of “unknown origin”.
The aim of the project
In our project we intend to create a copper biosensor, that would sense copper ions in growing media (based on a water sample). The advantage of biosensor over FAAS and MS techniques is, that it will sense true free copper concentration, available to biological system. It will not react to strongly ligand-bound copper and fine dispersed metallic copper.
To make our sensor more robust we intend to supplement it with a threshold device, that will provide the response on a “all or nothing” basis, when copper level will exceed the critical concentration. So, our project falls into two distinct parts. The first part is a copper-responsive element, that will convert measured copper level to POPS. The second part is a threshold device with hysteresis (a Schmitt trigger), that is designed as a individual part, so that it may be used with any input.
1. Copper responsive element
In the creation of copper responsive element we rely on natural copper sensing systems of E. coli Copper is the essential element for E. coli, so that its intracellular level is strictly regulated. Generally, outside copper concentration exceeds the copper need of the cell, so that excess copper is excreted. Intracellular copper level is sensed by CueR protein, that controls the expression of CopA efflux pump. We are not to affect this loopback, as malfunction of it will cause copper poisoning of the cell. We’d rather use another natural copper sensing loopback, that senses extracellular copper level. It consists of CusR/CusS two component signal system, that controls expression of CusCFBA operon, coding for copper efflux system. This system seems the most appropriate for our tasks. We intend to take the operator of CusCFBA operon as a copper sensing element. The possible problem is selectivity, as CusR/CusS specificity to copper is not well studied.
2. Schmitt trigger
In practice we often need a converter of gradual (analog) response to some signal to discrete (digital, all or nothing) response. In electronics this task is usually accomplished by building systems with positive feedback – comparators and Schmitt triggers. Comparators and Schmitt triggers share the same behavior, except for some major quantitative differences.
The ideal comparator should have two states - it should be in on state (logic 1), when the signal X is more than threshold level XT, and in off state (logic 0) when the signal is below X. This could be accomplished in electronics by using an amplifier with very high gain with its zero shifted to XT. Then both states correspond to negative and positive saturation of the device, or to saturation and cutoff of the device. Such device, however has little practical importance, because when input X, being changed, approaches XT, it can cause random oscillations of output, caused by instability and statistical nature of X. In electronics this effect is often caused “ringing”. That’s why the positive feedback is needed. It makes different the threshold levels transitions for low to high XT0→1 and from high to low XT1→0 so that XT0→1 > XT1→0. So the described system gets two stable states in the region (XT1→0, XT0→1) – it has a hysteretic behavior. The transitions, after they were initiated, become immune to further small changes of input signal. This approach decrease the accuracy of the comparator, but makes in relatively insensitive to ringing and oscillations. The hysteretic loop (XT1→0 - XT0→1) of a comparator is narrow (it is determined by the desired resolution).
The Schmitt trigger in fact is a comparator, but with a very wide hysteretic loop, that may comprise more than a half of dynamic range of the input signal. It is accomplished by very strong positive feedback. The width of hysteretic loop of a Schmitt trigger is often referred as “safety area”. All changes of the input signal, that fall inside the safety area are not sensed by the output. This is an ideal device to filter the signal with inherent ringing or excessive noise.
Implementations
Building effective comparators and Schmitt triggers meets several controversial requirements:
- the device must be stable in all the dynamic range, including hysteretic area
- the stationary states (on and off state) should be significantly different by output
- the off-on and on-off transitions should be fast, standard and well-triggered (no influence of input signal on output when transition has begun).
When implementing comparators and Schmitt triggers in gene regulatory systems we meet the problem, that didn’t allow us to use create direct analogs of comparators. Most simple comparators are built using bipolar power and differential amplifier, working in linear mode. The electronic comparator is most accurate if the threshold level is close to zero and becomes inoperational when we try to make threshold levels close to power source or sink voltages (saturation voltages). The principal differences of gene expression systems are:
- the transition characteristics of elements (repressors) are significantly nonlinear
- the phase space of the system (concentration of regulatory prioteins) is strictly positive
We perform a theoretical study, to find the best scheme with hysteresis (trigger).
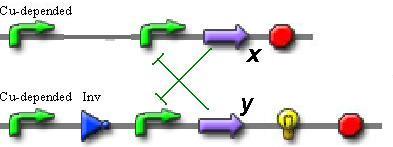
So, if we want to use only proteins without secondary metabolites, the best trigger sheme is the following:
In this scheme we can observe the switching between two repressors (X and Y), depending on the transcription level from the copper-depended promoter.
But this scheme is rather difficult and need many constructing operations. So, we decided to think more simple scheme, using the secondary metabolites.
The cross-repressor system: Theoretical studies
The system consists of two repressor genes, each gene repressed by the product of the other gene. It will be shown below, that, depending on the properties of operators, this system may be an amplifier, a comparator, a Schmitt trigger or a full trigger. Hysteretic behavior of this system may arise only if more than one repressor molecule binds the operator with positive cooperativity. The described system is probably the simplest, and it was not invented by us. λ-type bacteriophages use the very similar curcuit for establishing lysogeny in E.coli (the most well-known example is cI/cro repressor pair that makes a bistable trigger, corresponding to preservation and induction of λ-prophage).
Algebraic analysis of stability
The cross-repressor system: BioBrick implementation
We have searched the base for the proper components for our system and decided to use the folowing parts:
 |
| ||||||||||||||||||||||||||||||||||||
We have found two suitable repressor-operator systems B/PB(C0052/R0052 and C0053/R0053) and decided to use the second if something goes wrong with the first.
Anyway to date we have only managed to transform repressor of the first system and operator of the second into E.coli...