\Experimental Steps
From 2007.igem.org
(Difference between revisions)
| Line 105: | Line 105: | ||
<!-- PLACE CONTENT IN HERE--> | <!-- PLACE CONTENT IN HERE--> | ||
=Experiemental Steps= | =Experiemental Steps= | ||
| + | |||
==Step 1: AHL Assay== | ==Step 1: AHL Assay== | ||
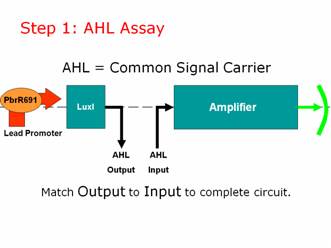
AHL is our common signal carrier between the two main components of our genetic circuit. The output coming from our lead promoter + LuxI is AHL. And the input going into the pLux part of our Amplifier is AHL. To make a working lead detector, the amount of AHL coming out of the first component must be sufficient to turn on the second component. Therefore, as in electrical engineering, we must measure and match our inputs and outputs to create a working genetic circuit. | AHL is our common signal carrier between the two main components of our genetic circuit. The output coming from our lead promoter + LuxI is AHL. And the input going into the pLux part of our Amplifier is AHL. To make a working lead detector, the amount of AHL coming out of the first component must be sufficient to turn on the second component. Therefore, as in electrical engineering, we must measure and match our inputs and outputs to create a working genetic circuit. | ||
| - | [[Image:step1lead.png]] | + | [[Image:step1lead.png|right]] |
We used the well characterized part BBa_T9002 for our AHL assay. These cells take in AHL and give us a straight GFP output, without amplification or a positive feedback loop. We created a calibration curve and planned to use these cells to test the amount of AHL coming OUT of our lead promoter in response to different amounts of lead. We would then separately test the amplifier by adding known concentrations of AHL to determine how much AHL is necessary to give us a GFP output. Then we can put both pieces of information together to give us a lead sensor.\ | We used the well characterized part BBa_T9002 for our AHL assay. These cells take in AHL and give us a straight GFP output, without amplification or a positive feedback loop. We created a calibration curve and planned to use these cells to test the amount of AHL coming OUT of our lead promoter in response to different amounts of lead. We would then separately test the amplifier by adding known concentrations of AHL to determine how much AHL is necessary to give us a GFP output. Then we can put both pieces of information together to give us a lead sensor.\ | ||
| Line 122: | Line 123: | ||
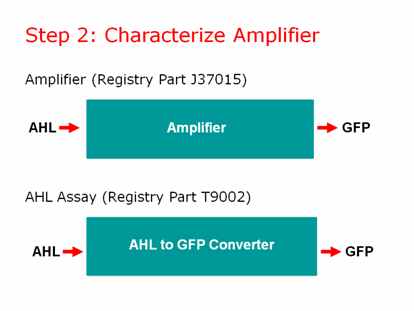
==Step 2: Characterize Amplifier== | ==Step 2: Characterize Amplifier== | ||
| - | [[Image:step2lead.png]] | + | [[Image:step2lead.png|right]] |
We characterized our amplifier and found some very unexpected results! | We characterized our amplifier and found some very unexpected results! | ||
Revision as of 01:45, 27 October 2007