Austin Day Notebook
From 2007.igem.org
My Construction Files
My Sequencing Files
AustinDay 15:17, 5 June 2007 (EDT)
- Designed the oligos for the hemoglobin mutants. Here are my notes on the mutants:
Primary Mutants:
Wild Type Hemoglobin
PPDA: P50 = 44, yield = 17.5%
Presbyterian mutation (Increase P50): Beta: Asn-108 -> Lys
Providence mutation (Increase yield): Beta: Lys-82 -> Asp
Di-alpha fusion (Increase yeidl): Glycine? linker.
P2996: P50 = 34, yield “average”
Presbyterian mutation (Increase P50): Beta: Asn-108 -> Lys
αL29F (Decrease P50, decrease auto-oxidation)
αV96W (Increase P50, increase auto-oxidation)
Oligo design for the following mutations:
ASN: AAC -> AAA
B-Asn108Lys
Lys AAG 14043.00 10.26 0.23
Lys AAA 46044.00 33.66 0.77
GAATTTTCGTCTGCTGGGTAAAGTGCTGGTGTGTGTCCTTGC
LYS: AAA -> GAT
B-Lys82Asp
Asp GAT 44103.00 32.24 0.63
Asp GAC 26201.00 19.15 0.37
CTGGCGCATCTTGATAATCTTGATGGTACATTCGCGACTCTGAG
Di-alpha fusion: Oligos coming soon.
LYS: TTA -> TTT
A-L29F
Phe TTT 30361.00 22.19 0.57
Phe TTC 22649.00 16.56 0.43
GAGTATGGTGCTGAAGCTTTTGAACGCATGTTTTTAAGCTTTC
VAL: GTT -> TGG
A-V96W
Trp TGG 20835.00 15.23 1.00
CGCATAAACTCCGTGTGGACCCGTGGAACTTTAAACTGCTGTCCCACTG
Secondary Mutants (Or mutations that would lead to them)
Alpha; VAL E11 -> Threonine: Increases P50 by 4x (to 25 torr). Due to stabilization of water molecule in distal heme pocket of decoy T-state. Increases auto-oxidation rate.
Beta; VAL E11 -> Threonine: Increases P50 by 2x (to 12 torr).
Alpha; V26T: 4x increase in P50. Lost cooperativity. (n = 1.1)
Beta; B67T: (n = 2.2) Slightly increased P50.
Combination of surface modifications to increase P50 by 3x:
o Val1 -> Met:
o His2 -> deleted
o Thr4 -> Ile
o Pro5 -> Ala
o Ala 76 -> Lys
βN108D, (In place of presbyterian) increases the P50 more than the presbyterian.
- In other news: All but I716053 #1 came out white. That colony was red, so I didn't mini it. (parent vector)
- I'm going to go on with the construction now with the RBs libraries from yesterday.
- I used the #1 clone from each of the parts for the next digest/ligation/transformation round.
- I transformed the colonies with the RBS library. I forgot to select for the secondary antibiotic, but Chris said I should just scrape the plate tomorrow and grow it all up in the antibiotic marker. Hopefully that'll do the trick.
AustinDay 13:25, 4 June 2007 (EDT)
- Got a nice number of colonies on the synthetic gene biobrick transformations from yesterday. I grew up 2 of each.
- I minied the RBS library parts (I716051 and I716052).
AustinDay 16:50, 3 June 2007 (EDT)
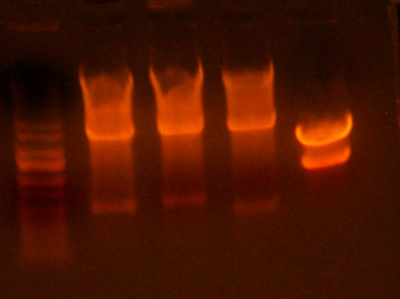
- Made an EcoRI / XhoI digest of the synthetic genes and a digest of EcoRI and XhoI of the 9145 plasmid.
- Here's the gel. Very pretty.
- Took the small fragment from the synthetic genes and the larger fragment from the 9145 plasmid and ligated them together.
- I think I'm going to name these new parts: I716053 (9186 + 9145), I716054 (9187 + 9145), I716055 (9188 + 9145). These names came to me in a dream. It was said they shall bring luck and prosperity to all who use them.
- I grew up 2 colonies of the homogenized RBS pools. (The Kan and the Cmr pools)
Austinday 15:43, 2 June 2007 (PDT)
- Okay, so since the 1121 part was bad, chris found a PCR product of it. I'm going to give that a try and see if it works.
- I did the ligation and transformation for those. I named the parts Bad0001 (kan) and Bad0002 (Cmr). Plated and cooking for tomorrow.
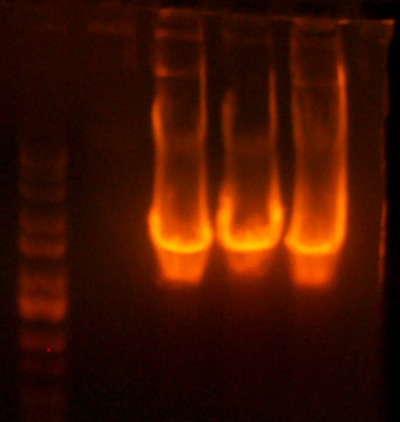
- Made digest of BglII and XhoI of the 3X concentrated 9145 plasmid miniprep. The total digest was equal to that of 14 regular digests. The gel is below:
- Digested the DNA 2.0 plasmids with EcoR1 and XhoI. But...the gel looks funny. I may not have allowed enough time for the lyophilized DNA to redissolve... That's my best guess at least.
- I grew up some colonies of the DNA 2.0 parts so I'll give it another try tomorrow. (Chris told me that there is probably too much DNA and that if I ran it longer, it might have cleared up. You can kind of see that there might be a second band...sort of.)
Austinday 22:59, 30 May 2007 (PDT)
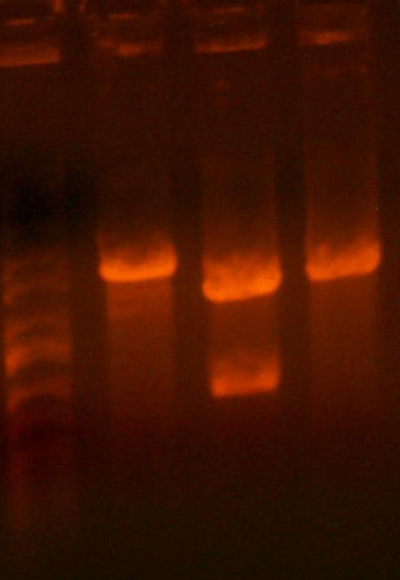
- Okay, I repeated the digestion from yesterday, but got the same results. Here's the gel.
- This time, I let it digest for well over an hour and mixed it half way through. I also ran the gel at a lower voltage than usual.
- The bands for the 1122 lane was noticeably cleaner, but the 1121 lane was uncut.
- I cut out the smaller band from the 1122 lane(because it's probably a higher yield than the previous gel), as well as the 1128 band. I figured that because the previous gel didn't cut completely, and because the 1128 pool smaller bands would have already ran off the gel, this second gel would have a more pure band than the first. I also cut out the region of the 1121 lane where the smaller band should be, although it looks as if nothing is there... I guess we'll have to remake that 1121 part.
Austinday 16:53, 29 May 2007 (PDT)
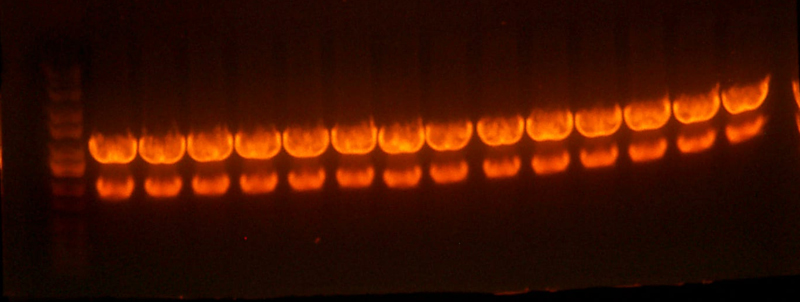
- Sequencing of the pBca1101-Bca1128 RBS library resulted in 2 duplicate RBS's. #24 was identical to #3, and #5 was identical to #2 so I threw out #3 and #5.
- I pooled together the bca1128 rbs's and the 1106 A and B rbs's as well as one other 1128 A rbs Chris had.
- I digested those with EcoR1 and BglII to create the vectors to insert the antibiotic markers. After digestion, the pool looked good so I cut it out right away. The 1121 and 1122 (CmR and Kan) digestions didn't look correct.
- The lanes are: Marker (Barely came out), 1121, 1122, and rbs pool (cut out)
- 1121 has the right number of bands, but I can't exactly tell if the size of the smaller band is correct.
- 1122 has the wrong number of bands. I'm not that experienced with picking out when a band is undigested plasmid, but even if that were the case for the third band, the smallest band looks much too small.
- Anyway, I cut out the smallest bands from 1121 and 1122 and stored them for tomorrow. Maybe Chris will have something to add to my gelatinous adventures.
198.128.27.101 13:55, 25 May 2007 (PDT)
- Preliminary ranking of RBS's picked from the library based on culture colors: (Bca1101 - Bca1128's in TG1)
Stronger --> Weaker
24, 1, 10, 18, 2, 3, 5, 9, 19, 4, 12, 11, 20, 21, 16, 13, 17, 7, 6, 15, 22, 14, 23
The first 7 are significantly more red than the rest.
# 11 was lost due to a tragic mini prepping accident. May it rest in peace.
AustinDay 16:38, 3 June 2007 (EDT)
- Time to start some awesomeness.
- Biobrick numbers: 051-100 Austin Day
- The next few entries are cut and pasted from my Arkin Wiki because I started making entries on that before this wiki was set up.