Boston University
From 2007.igem.org
| Line 1: | Line 1: | ||
[[Image:Rhett2.JPG|center|500px]] | [[Image:Rhett2.JPG|center|500px]] | ||
| - | |||
| - | |||
== About Us == | == About Us == | ||
Revision as of 20:03, 1 July 2007
About Us
Welcome to the wiki for Boston University's iGEM 2007 team!
Our team consists of David Shi, Rahul Ahuja, Christian Ling, and Danny Bellin, all soon-to-be juniors majoring in Biomedical Engineering at Boston University.
We are advised by [http://www.bu.edu/dbin/bme/faculty/?prof=tgardner Dr. Timothy Gardner], Assistant Professor of Biomedical Engineering, as well as Frank Juhn, Kevin Litcofsky, and Stephen Schneider, students in the [http://gardnerlab.bu.edu/ Gardner Laboratory], where we work. We are grateful to our advisors for their time and support!
We are also grateful to [http://www.pfizer.com Pfizer], the [http://www.bu.edu/eng Boston University College of Engineering], and the [http://www.bu.edu/eng/bme Boston University Department of Biomedical Engineering], for their generous support of our team.
Our Project Plan
Electrogenic microbes are microscopic organisms capable of extracting electrons from organic or chemical material and passing these electrons to extracellular organic or inorganic substrates. The continued extraction and transfer of these electrons results, by definition, in usable electric current. While electrogenic capacities have been observed among multiple bacteria species, Shewanella oneidensis is of particular interest due to its ability to oxidize virtually any carbon source. Research is currently underway into exploiting S. oneidensis for microbial fuel cells driven by ubiquitous material like waste and cellulose.
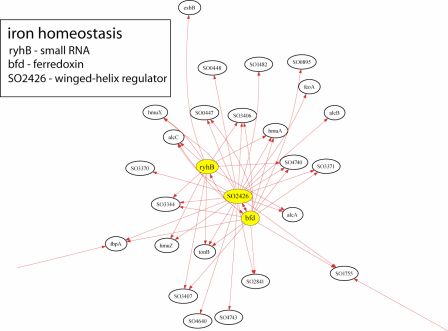
S. oneidensis-based fuel cells will be improved if the microbe’s own electrogenic capability is improved through manipulation of genes controlling current production. The main problem, however, is determining how genetic modifications will affect an organism, as the interactions among genes in a single organism form a highly complex circuit network that is not readily resolved. To address this problem, Dr. Timothy Gardner and his lab at Boston University have developed an algorithm called CLR to map the transcriptional regulatory interactions in microbial genomes. Once CLR produces a map, effects of genetic engineering will be much more predictable, and those modifications leading to the most beneficial result will be easier to identify. The following is an example of a transcriptional network inferred by CLR for just one of S. oneidensis’s metabolic processes.
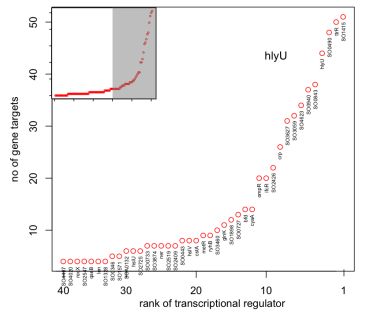
The Gardner Laboratory has used CLR to map 800 regulatory interactions controlling metabolism and electron transport in S. oneidensis. Results from the work have revealed that the transcription regulators SO1415, ttrR, and hlyU affect the largest number of genes involved in these processes:
Because of their global regulatory role, we hypothesize that mutations of these three genes present a high chance of affecting S. oneidensis in significant ways and potentially enhancing electrogenic output.
Our plan so far:
- Transform mutated global transcription factors
- Choose plasmids
- Choose restriction enzymes
- Design primers for amplification
- Perform error-prone PCR
- Transform mutated genes into E. coli
- Restriction enzyme digestion
- Ligation
- Transformation into E. coli
- Conjugate E. coli with Shewanella
- Screen/select Shewanella strains for increased current production due to mutations. Potential methods:
- Alginate (?) beads and fluorocytometer
- Metallo-Antibiotics
- (DB's random idea): Could we take advantage of spectrophotometry? Perhaps we could split our collection of mutants into different groups, measure their absorbances with spectrophotometry, and assume that the sample with the lowest absorbance contains mutants producing more electricity and therefore growing slower. We could then split this sample into different groups and repeat. While there might be some inefficient strains in the successful broth samples, on the whole, the broth might be a good one for use in a fuel cell. Problem: Low absorbance could be due to mutants losing viability. Potential Solution: Let initial sample grow for a while so all mutants unable to grow will die off, all mutants able to grow will thrive, and then perform the screen.