Boston University/Electroporation Results
From 2007.igem.org
< Boston University(Difference between revisions)
| (One intermediate revision not shown) | |||
| Line 49: | Line 49: | ||
==Overview of Electroporation Results== | ==Overview of Electroporation Results== | ||
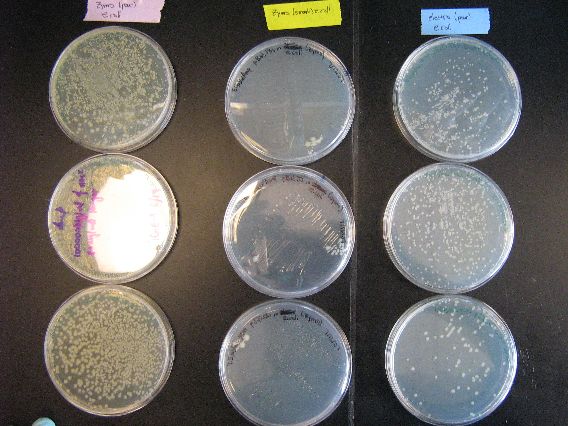
| - | :The results for electroporation were very encouraging. In E. coli control plates, there were plenty of colonies for all three plates (first picture, right column) which indicates that all three plasmids were successfully transformed into E. coli. Another important detail from the results is that the pj200 plate (bottom of the right column in the first picture) has considerably less colonies than the other two plates. This means that the transformation efficiency of pjq200 is less than the two pBAD plasmids (the results of the [[Boston_University/ | + | :The results for electroporation were very encouraging. In E. coli control plates, there were plenty of colonies for all three plates (first picture, right column) which indicates that all three plasmids were successfully transformed into E. coli. Another important detail from the results is that the pj200 plate (bottom of the right column in the first picture) has considerably less colonies than the other two plates. This means that the transformation efficiency of pjq200 is less than the two pBAD plasmids (the results of the [[Boston_University/Zymo_Transformation_Results | Zymo transformation]] also suggest this). |
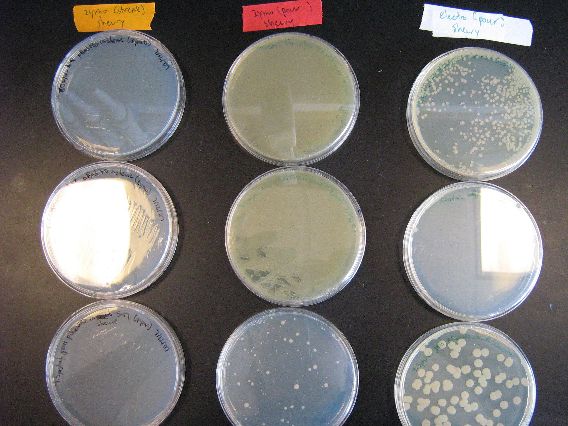
:More importantly, electroporation into Shewy ended up being successful for two out of three plasmids (second picture, right column). Plasmids pBAD18s and pjq200 both had many colonies and have both been proven capable of electroporation into S. oneidensis. The complete lack of cells in the Shewy/pBAD30 plate, however, is a bit baffling as electroporation of pBAD30 working with the E. coli control AND Zymo transformation of pBAD30 into E. coli is successful. Further testing of the combination of pBAD30, Shewy, and electroporation will have to be conducted to definitively determine whether or not PBAD30 is capable of electroporation into Shewy. | :More importantly, electroporation into Shewy ended up being successful for two out of three plasmids (second picture, right column). Plasmids pBAD18s and pjq200 both had many colonies and have both been proven capable of electroporation into S. oneidensis. The complete lack of cells in the Shewy/pBAD30 plate, however, is a bit baffling as electroporation of pBAD30 working with the E. coli control AND Zymo transformation of pBAD30 into E. coli is successful. Further testing of the combination of pBAD30, Shewy, and electroporation will have to be conducted to definitively determine whether or not PBAD30 is capable of electroporation into Shewy. | ||
| - | + | <!-- | |
[[Image:BU_electro_pour_ecoli_pbad18s.jpg]] | [[Image:BU_electro_pour_ecoli_pbad18s.jpg]] | ||
[[Image:BU_electro_pour_ecoli_pbad18s_flash.jpg]] | [[Image:BU_electro_pour_ecoli_pbad18s_flash.jpg]] | ||
| Line 66: | Line 66: | ||
[[Image:BU_shewy_zymo_electro.jpg]] | [[Image:BU_shewy_zymo_electro.jpg]] | ||
| + | !--> | ||
[[Boston University | Back]] | [[Boston University | Back]] | ||
Latest revision as of 16:42, 20 July 2007
Introduction to Electroporation and Testing
- During the weekend of 7/14/07, the BU iGEM team ran a test of Electroporation of plasmids pBAD18s, pBAD30, and pjQ200 into wildtype S. oneidensis. Plasmids pBAD18s and pBAD30 were recommended to us by Professor Gardner as possible vectors for mutated Shewy genes. pjQ200 was the plasmid our team had previously selected as a prime candidate for serving as a vector. In order to check (and in the case of pjQ200, double-check) that these plasmids are capable of direct transformation into Shewy, tests of Zymo transformation and electroporation were conducted. For both types of transformation, E. coli was used as a control to verify that our transformation procedures were conducted properly. The results are posted below in a series of images and a short analysis. Lastly, gentamycin plates were used to select for pjq200 transformants, while ampicillin plates were used to select for pBAD18s and pBAD30 transformants.
Results of Electroporation
Overview of Electroporation Results
- The results for electroporation were very encouraging. In E. coli control plates, there were plenty of colonies for all three plates (first picture, right column) which indicates that all three plasmids were successfully transformed into E. coli. Another important detail from the results is that the pj200 plate (bottom of the right column in the first picture) has considerably less colonies than the other two plates. This means that the transformation efficiency of pjq200 is less than the two pBAD plasmids (the results of the Zymo transformation also suggest this).
- More importantly, electroporation into Shewy ended up being successful for two out of three plasmids (second picture, right column). Plasmids pBAD18s and pjq200 both had many colonies and have both been proven capable of electroporation into S. oneidensis. The complete lack of cells in the Shewy/pBAD30 plate, however, is a bit baffling as electroporation of pBAD30 working with the E. coli control AND Zymo transformation of pBAD30 into E. coli is successful. Further testing of the combination of pBAD30, Shewy, and electroporation will have to be conducted to definitively determine whether or not PBAD30 is capable of electroporation into Shewy.