Paris/July 23
From 2007.igem.org
(→PCRs) |
(→PCRs) |
||
| Line 191: | Line 191: | ||
|Band= | |Band= | ||
|}} | |}} | ||
| + | |||
| + | == PCR mutagenesis DGAT == | ||
{{Paris_PCR_0| Title = DGAT1 | {{Paris_PCR_0| Title = DGAT1 | ||
| Line 211: | Line 213: | ||
|Band= | |Band= | ||
|}} | |}} | ||
| - | + | <br> | |
{{Paris_PCR_0| Title = DGAT2 | {{Paris_PCR_0| Title = DGAT2 | ||
| Line 232: | Line 234: | ||
|Band= | |Band= | ||
|}} | |}} | ||
| + | <br> | ||
== E.Coli pKS::DGAT == | == E.Coli pKS::DGAT == | ||
Revision as of 10:17, 31 July 2007
Contents |
w121 growth
w121 culture launched on 22/07/07 did not grow ON: I had forgot to add DAP to LB-Erythro growth medium.
at 12h30, the following culture was launched:
- w121 in 2ml LB-Erythro-DAP
We were unable to perform growth kinetics analysis of w121 in different DAP-supplemented media (because the machine is occupied for the night
Transduction of DapA deletion in MG1655
- The transduction seems to have worked: isolation of clones on LB and LB+DAP for verification.
Migration of digestion products
MiniPrep
Digestion reactions
Each of the digestion reactions that follow were performed as follows:
- 20µL DNA solution (miniprep or PCR product)
- 5µL Buffer 10x
- 0.5µL BSA 100x
- 2µL of each of the 2 enzymes indicated
- H20 qsp 50µL
NEB3 buffer used for XbaI/PstI double digestion NEBEcoRI buffer used for both EcoRI/SpeI & EcoRI/PstI double digestion reactions
| Digestion Products | ||||||||
|---|---|---|---|---|---|---|---|---|
| Number | Product Name | Matrix Name | Enzyme 1 | Enzyme 2 | size | Description | ||
| D22 | pSB1A2 open vector | BBa_J61047 (MP9.1 & MP9.2) | EcoI | PstI | Open pSB1A2 vector for BioBrick EcoRI/PstI insertio | |||
| D23 | AraC/pBad promoter fw-insert | BBa_I0500 (MP4.1 & MP4.2) | EcoI | SpeI | AraC/pBad promoter ready for use as a forward insert | |||
| D24 | eCFP bw-insert | BBa_E0422 (MP6.1 & MP6.2) | XbaI | PstI | eCFP (RBS-OFR_LVA-T) ready for use as a backward insert | |||
| D25 | PoPs to GFP converter | BBa_E0421 (MP7.1 & MP7.2) | XbaI | PstI | PoPs to GFP converter ready for use as a backward insert | |||
| D26 | gfp-tripart | BBa_E0840 (MP8.1 & MP8.2) | XbaI | PstI | gfp-tri part (strongRBS-ORF-T) ready for use as a backward insert | |||
| D27 | lox71-ftsZ bw-insert | lox71-ftsZ PCR product P7 | XbaI | PstI | lox71-ftsZ(unmutated) ready for use as a backward insert | |||
PCRs
| PCR : Lox71-FtsA-FtsZ | ||||||
|---|---|---|---|---|---|---|
| PCR Settings | Buffer (5x) | 5x 10µL | Expected size | |||
| Annealing (°C) | MgCl2 10µM | 10µM 0µL | 2058 | |||
| 60 | dNTP 10µM | 10µM 1µL | Success | |||
| Time Elongation | Oligo F 10µM | 3 Lox71-FtsA-F | 2.5µL | |||
| 3m00' | Oligo R 10µM | 2 FtsZ-R | 2.5µL | Image (click to enlarge) | ||
| Number cycles | Water | 34µl | [[Image:|30px]] | |||
| 35 | Polymerase | Phusion 0.5µL | Band (0=ladder) | |||
| DNA | Toothpick in MG16655 Glycerol | |||||
| PCR : Assembly PCR Lox71-FtsA-FtsZ-1 + FtsZ-2 | ||||||
|---|---|---|---|---|---|---|
| PCR Settings | Buffer (5x) | 5x 10µL | Expected size | |||
| Annealing (°C) | MgCl2 10µM | 10µM 0µL | 2058 | |||
| 70°C (5x) without oligos + 60°C (35x) | dNTP 10µM | 10µM 1µL | Success | |||
| Time Elongation | Oligo F 10µM | 3 Lox71-FtsA-F | 2.5µL | |||
| 3m00' | Oligo R 10µM | 2 FtsZ-R | 2.5µL | Image (click to enlarge) | ||
| Number cycles | Water | 34µl | [[Image:|30px]] | |||
| 40x | Polymerase | Phusion 0.5µL | Band (0=ladder) | |||
| DNA | 8.5µl Lox71-FtsA-FtsZ-1 (~50ng) + 17.5µl FtsZ-2 (~50ng) | |||||
| PCR : B0030-DapAColi | ||||||
|---|---|---|---|---|---|---|
| PCR Settings | Buffer (5x) | 5x 10µL | Expected size | |||
| Annealing (°C) | MgCl2 10µM | 10µM 0µL | 952 | |||
| 60°C | dNTP 10µM | 10µM 1µL | Success | |||
| Time Elongation | Oligo F 10µM | o20 RBS-DapAColi | 2.5µL | |||
| 3m00' | Oligo R 10µM | o7 DapAColi-R | 2.5µL | Image (click to enlarge) | ||
| Number cycles | Water | 34µl | [[Image:|30px]] | |||
| 35x | Polymerase | Phusion 0.5µL | Band (0=ladder) | |||
| DNA | Toothpick in MG16655 Glycerol | |||||
| PCR : B0030-DapASubtilis | ||||||
|---|---|---|---|---|---|---|
| PCR Settings | Buffer (5x) | 5x 10µL | Expected size | |||
| Annealing (°C) | MgCl2 10µM | 10µM 0µL | 946 | |||
| 60°C | dNTP 10µM | 10µM 1µL | Success | |||
| Time Elongation | Oligo F 10µM | o20 RBS-DapASubtilis | 2.5µL | |||
| 3m00' | Oligo R 10µM | o9 DapASubtilis-R | 2.5µL | Image (click to enlarge) | ||
| Number cycles | Water | 34µl | [[Image:|30px]] | |||
| 35x | Polymerase | Phusion 0.5µL | Band (0=ladder) | |||
| DNA | Toothpick in MG16655 Glycerol | |||||
PCR mutagenesis DGAT
| PCR : DGAT1 | ||||||
|---|---|---|---|---|---|---|
| PCR Settings | Buffer (5x) | 5x 10µL | Expected size | |||
| Annealing (°C) | MgCl2 10µM | 10µM 0µL | ||||
| 50 | dNTP 10µM | 10µM 1µL | Success | |||
| Time Elongation | Oligo F 10µM | 27 ForDGAT1 | 2.5µL | |||
| 2m00' | Oligo R 10µM | 13 DPst1-DGAT-R | 2.5µL | Image (click to enlarge) | ||
| Number cycles | Water | 33µl | [[Image:|30px]] | |||
| 35 | Polymerase | Phusion 0.5µL | Band (0=ladder) | |||
| DNA | Miniprep pKS::DGAT (1µL = 138ng) | |||||
| PCR : DGAT2 | ||||||
|---|---|---|---|---|---|---|
| PCR Settings | Buffer (5x) | 5x 10µL | Expected size | |||
| Annealing (°C) | MgCl2 10µM | 10µM 0µL | ||||
| 50 | dNTP 10µM | 10µM 1µL | Success | |||
| Time Elongation | Oligo F 10µM | 12 DPst1-DGAT | 2.5µL | |||
| 2m00' | Oligo R 10µM | 27 Rev-DGAT1 | 2.5µL | Image (click to enlarge) | ||
| Number cycles | Water | 33µl | [[Image:|30px]] | |||
| 35 | Polymerase | Phusion 0.5µL | Band (0=ladder) | |||
| DNA | Miniprep pKS::DGAT (1µL = 138ng) | |||||
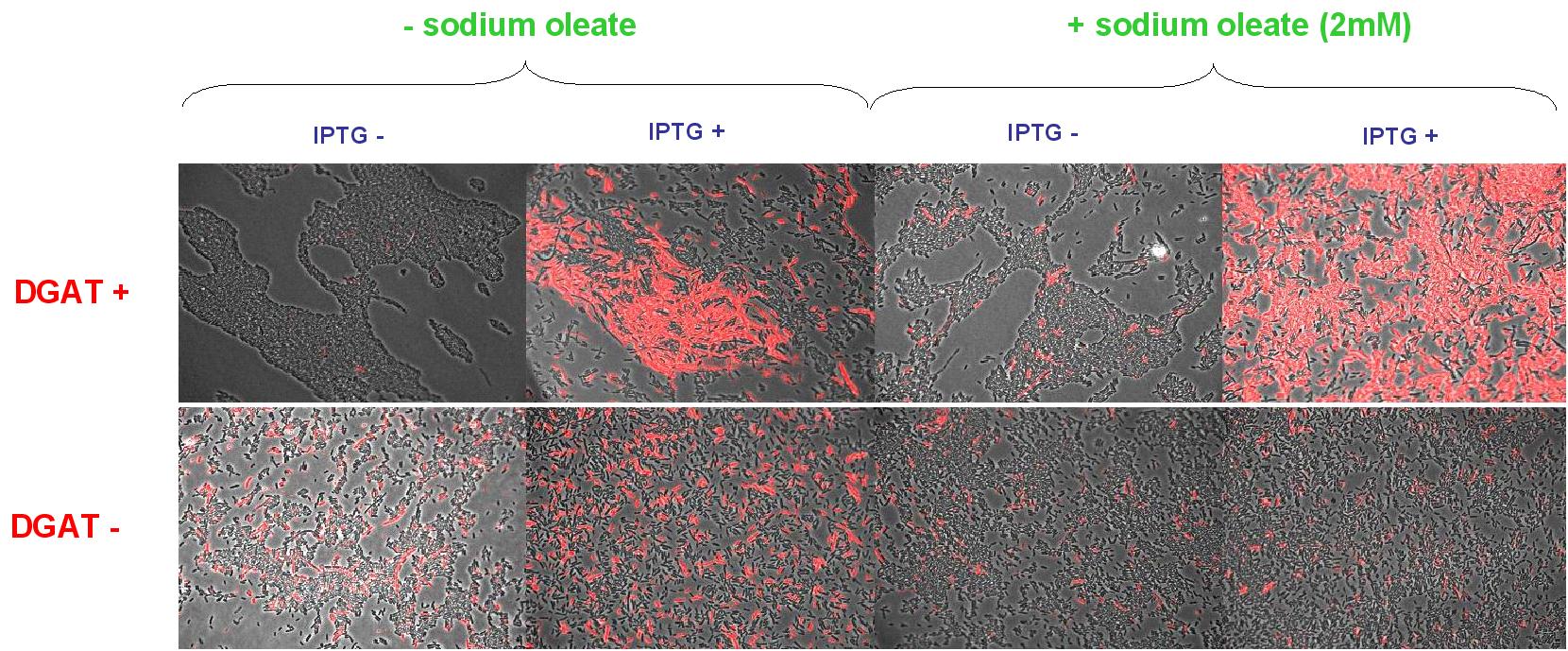
E.Coli pKS::DGAT
We look under microscopy 5 days after incubation of E.coli transformed by pKS::DGAT and the control E.coli transformed by part B0015 on different LB medium (See July 18).
- Observation:
We can observe E.coli single cells (100X).
- Interpretation:
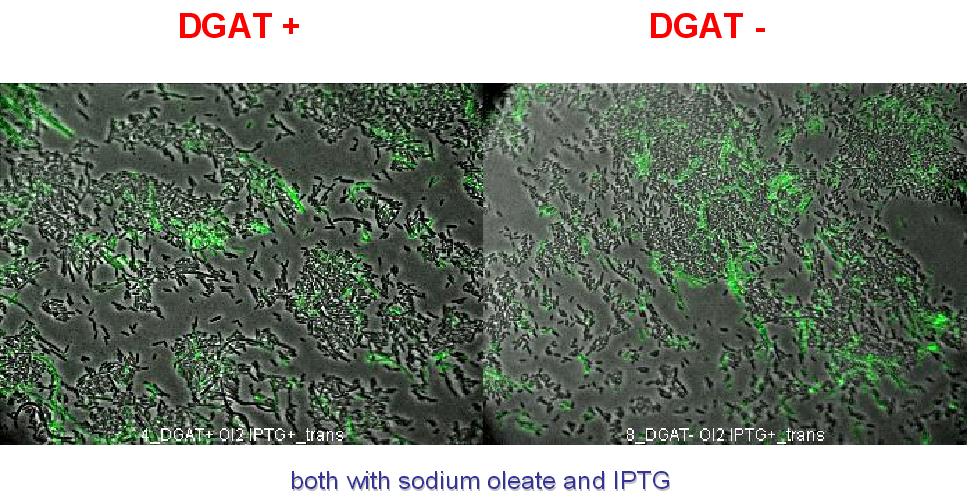
We can observe lipid inclusion into E.coli transformed by pKS::DGAT with IPTG induction. With or without adding oleate we do not observe significant differences. We could think that only dead cells are fluorescent (DGAT could kill the cells) but with cell death marker (green) we can see that cell death is not increased with DGAT.