The Goal
From 2007.igem.org
| Line 26: | Line 26: | ||
== '''Bottom-up biology: A proof of principle''' == | == '''Bottom-up biology: A proof of principle''' == | ||
| + | |||
| + | [[image:loops1.png|400 px|right|Bottom-up biology]] | ||
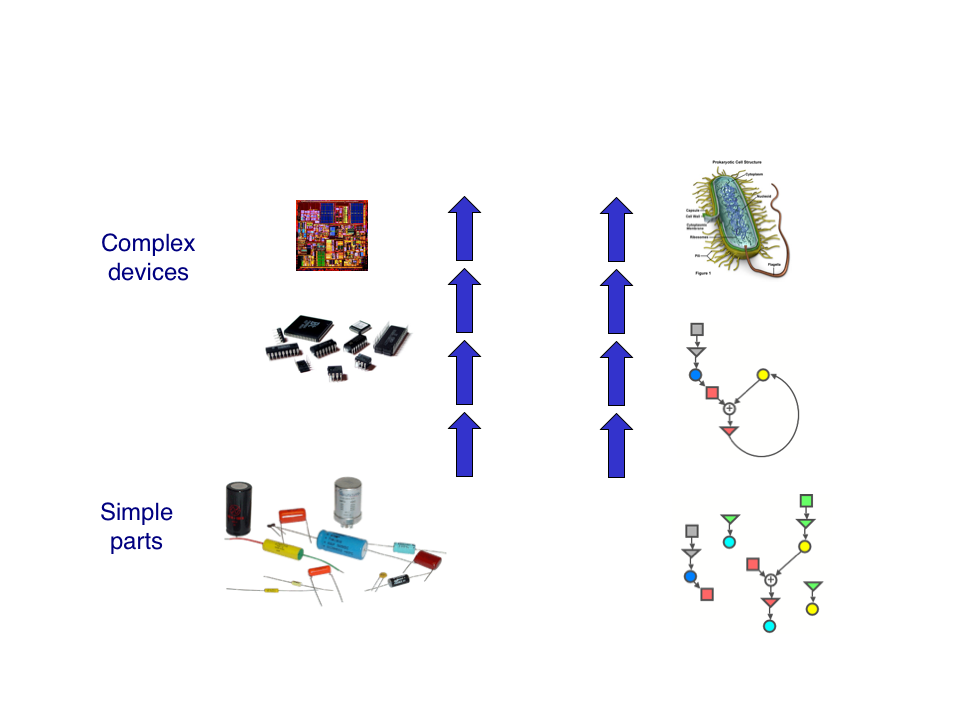
As synthetic biologists, we aim to build biological systems from the bottom-up. The general idea is that, by measuring the behavior of simple parts, we should be able to design and construct complex systems from those parts. But can this be done in practice? | As synthetic biologists, we aim to build biological systems from the bottom-up. The general idea is that, by measuring the behavior of simple parts, we should be able to design and construct complex systems from those parts. But can this be done in practice? | ||
| - | |||
| - | |||
We set out to test this idea rigorously. Our project is designed so that, by measuring the characteristics of relatively simple devices called 'open-loops', we are able to predict the responses of more complex systems called 'closed-loops'. These predictions can be directly tested against the observed responses. If we are successful, it would represent the first experimental validation of this powerful bottom-up design principle. | We set out to test this idea rigorously. Our project is designed so that, by measuring the characteristics of relatively simple devices called 'open-loops', we are able to predict the responses of more complex systems called 'closed-loops'. These predictions can be directly tested against the observed responses. If we are successful, it would represent the first experimental validation of this powerful bottom-up design principle. | ||
| Line 41: | Line 41: | ||
=== '''From open-loop characteristics to closed-loop responses''' === | === '''From open-loop characteristics to closed-loop responses''' === | ||
| + | [[image:loops2.png|400 px|right|From open loops to closed loops]] | ||
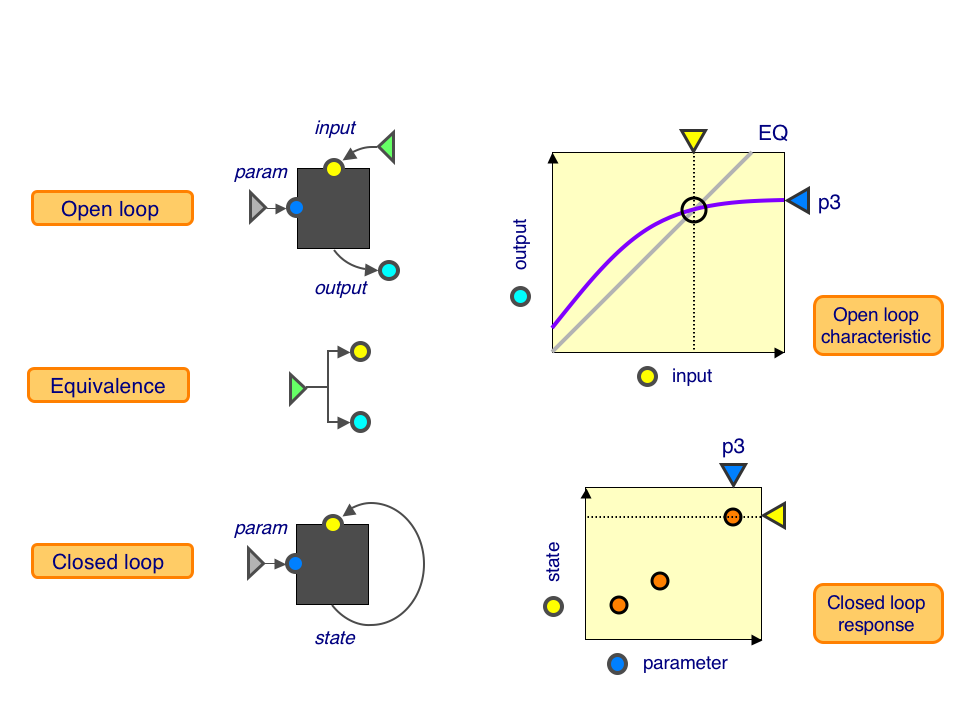
| - | + | Open loop (top): This is a a black box with a parameter knob (blue), an input (yellow) and an output (cyan). Keeping the parameter fixed, we can dial up the input and measure the resulting outputs, giving us the open-loop characteristic (purple curve). | |
| - | + | Equivalence (middle): This tells us precisely how much output corresponds to a given amount of input, shown as the equivalence line (grey line). | |
| + | |||
| + | Closed loop (bottom): This is a black box with a parameter knob (blue) and a state (yellow). We can vary the parameter values and measure the resulting state, giving us the closed-loop response. | ||
| + | |||
| + | The point where the open-loop characteristic intersects the equivalence line is a self-consistent state: the given output, when fed back through the system, returns precisely the same output. At this point, the open-loop and closed-loop systems look identical. By measuring these intersections as the parameter is varied, we can predict the closed-loop response from open-loop characteristics. | ||
| + | |||
| + | === '''Genetic implementation of open loops and closed loops''' === | ||
| + | |||
| + | [[image:loops3.png|400 px|right|Genetic implementations of loops]] | ||
| + | |||
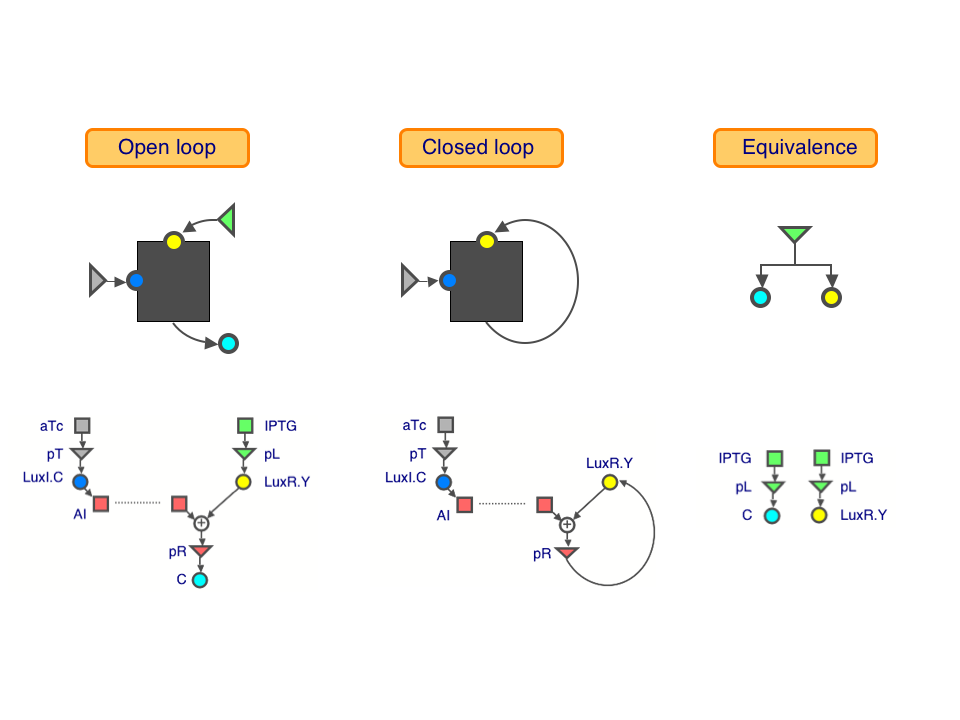
| + | We used three transcriptional regulatory modules to build our loops: the IPTG-inducible lac system, the aTc-inducible tet system, and the lux quorum-sensing system of Vibrio fischeri. | ||
| + | |||
| + | |||
| + | =='''References'''== | ||
| - | + | David Angeli, James E. Ferrell, Jr., and Eduardo D. Sontag (2004). Detection of multistability, bifurcations, and hysteresis in a large class of biological positive-feedback systems. '''PNAS''', 101, 1822 – 1827. | |
Revision as of 07:04, 21 October 2007
| On June 1st 2007, a group of undergraduate of various disciplines and from around the country assembled at NCBS, to attempt a 'proof of principle' demonstration. Their mission: to assemble and test a 'genetically engineered machine', a complex network assembled from simple biological parts. You will be amazed how much they managed to achieve in just six weeks! | |
|
The National Centre for Biological Sciences is a research institute located in Bangalore, India. In 2006, a team of graduate students from NCBS was the first from India to participate in iGEM. This year, we are very excited to compete as an all-undergraduate team of six students, from six different colleges located in four cities around India. Now that the summer is over, we hope hope to take the excitement of iGEM back to our home institutions, and create new teams across India who will participate in future iGEMs. | |
| National Centre for Biological Sciences, Bangalore |
| Home | The Team | The Goal | Parts Data Sheets | Mathematical Models | e-Notebook |
|---|
Contents |
Bottom-up biology: A proof of principle
As synthetic biologists, we aim to build biological systems from the bottom-up. The general idea is that, by measuring the behavior of simple parts, we should be able to design and construct complex systems from those parts. But can this be done in practice?
We set out to test this idea rigorously. Our project is designed so that, by measuring the characteristics of relatively simple devices called 'open-loops', we are able to predict the responses of more complex systems called 'closed-loops'. These predictions can be directly tested against the observed responses. If we are successful, it would represent the first experimental validation of this powerful bottom-up design principle.
Design: from parts to systems
One way to design a system is to build a detailed mathematical model. However, even the simplest model will contain many parameters whose values are unknown (for example, we have built a model with three dynamical variables, but 10 dimensionless parameters). We could imagine carefully designing experiments to extract all these parameter values, but once the system becomes moderately large, this becomes impractical. What we need is an approach that scales up easily.
Suppose that we are interested in only certain aspects of system behavior. In this case, it is not necessary to pin down every parameter value. By making just a few clever measurements, we can extract all the information we need. For example, recent theoretical work [Angeli et al., 2004] has shown that the steady state response of a system involving feedback ('closed loop') can be inferred by characterizing its response when feedback is cut ('open loop'). This technique can be applied to arbitrarily complex systems, as long as they satisfy certain simple conditions -- it scales up.
From open-loop characteristics to closed-loop responses
Open loop (top): This is a a black box with a parameter knob (blue), an input (yellow) and an output (cyan). Keeping the parameter fixed, we can dial up the input and measure the resulting outputs, giving us the open-loop characteristic (purple curve).
Equivalence (middle): This tells us precisely how much output corresponds to a given amount of input, shown as the equivalence line (grey line).
Closed loop (bottom): This is a black box with a parameter knob (blue) and a state (yellow). We can vary the parameter values and measure the resulting state, giving us the closed-loop response.
The point where the open-loop characteristic intersects the equivalence line is a self-consistent state: the given output, when fed back through the system, returns precisely the same output. At this point, the open-loop and closed-loop systems look identical. By measuring these intersections as the parameter is varied, we can predict the closed-loop response from open-loop characteristics.
Genetic implementation of open loops and closed loops
We used three transcriptional regulatory modules to build our loops: the IPTG-inducible lac system, the aTc-inducible tet system, and the lux quorum-sensing system of Vibrio fischeri.
References
David Angeli, James E. Ferrell, Jr., and Eduardo D. Sontag (2004). Detection of multistability, bifurcations, and hysteresis in a large class of biological positive-feedback systems. PNAS, 101, 1822 – 1827.