UCSF/Cloning Strategy1
From 2007.igem.org
< UCSF(Difference between revisions)
| (4 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | '''1- Cloning Strategy | + | '''1- Cloning Strategy''' |
| - | a- | + | :'''a- Multi-part Cloning of Plasmid-Encoded Promoters, Negative Effectors and Recruitment Domains''' |
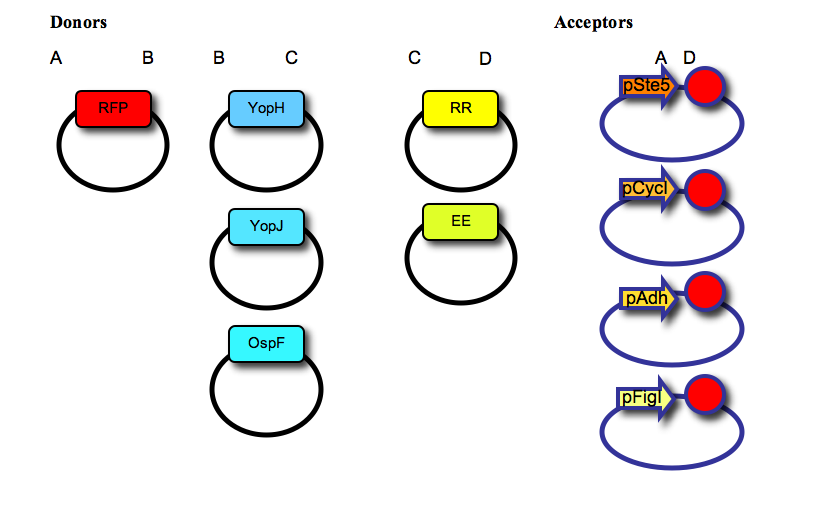
| - | We have used a multipart cloning strategy based on the Type IIs restriction enzyme AarI to generate, in a single ligation, a vector containing: a promoter, a fluorescent protein tag, a negative effector, a recruitment domain (synthetic leucine zipper) and a transcription terminator. For that, we first made two families of “Donor” and “Acceptor” vectors, based on the yeast expression vector pRS315. The 5 donor vectors contain a single part (RFP tag; negative effectors YopH, YopJ or OspF; synthetic leucine zippers for recruitment, “EE” or “RR”) cloned between two AarI sites, with distinct 4-base overhangs (named A, B, C, or D, as shown in the Figure below). The 4 acceptor vectors contain a promoter (pSte5, pCyc, pAdh, or pFig; the first three are constitutive with low, medium or high expression levels respectively, the last one is induced by activation of the mating pathway), two AarI restriction sites (each one with a distinct 4-base overhang, named A and D), and a transcription terminator (red circle); as shown in the figure below:''' | + | :'''We have used a multipart cloning strategy based on the Type IIs restriction enzyme AarI to generate, in a single ligation, a vector containing: a promoter, a fluorescent protein tag, a negative effector, a recruitment domain (synthetic leucine zipper) and a transcription terminator. For that, we first made two families of “Donor” and “Acceptor” vectors, based on the yeast expression vector pRS315. The 5 donor vectors contain a single part (RFP tag; negative effectors YopH, YopJ or OspF; synthetic leucine zippers for recruitment, “EE” or “RR”) cloned between two AarI sites, with distinct 4-base overhangs (named A, B, C, or D, as shown in the Figure below). The 4 acceptor vectors contain a promoter (pSte5, pCyc, pAdh, or pFig; the first three are constitutive with low, medium or high expression levels respectively, the last one is induced by activation of the mating pathway), two AarI restriction sites (each one with a distinct 4-base overhang, named A and D), and a transcription terminator (red circle); as shown in the figure below:''' |
| - | [[Image:DonorAcceptors.png]] | + | <center>[[Image:DonorAcceptors.png]]</center> |
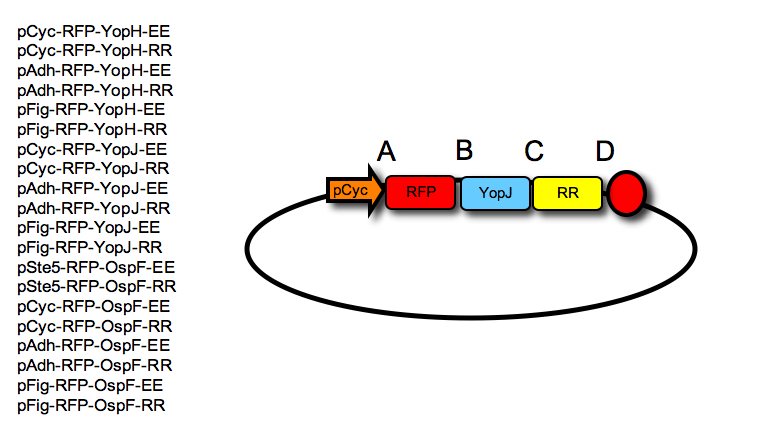
| - | '''Each final construct was then made by ligation into a given acceptor vector, an RFP tag, followed by a negative effector and a leucine zipper, resulting in the following constructs (one example shown in the Figure):''' | + | :'''Each final construct was then made by ligation into a given acceptor vector, an RFP tag, followed by a negative effector and a leucine zipper, resulting in the following constructs (one example shown in the Figure):''' |
| + | |||
| + | <center>[[Image:Plasmidcomp.png]]</center> | ||
| + | |||
| + | :'''b- Cloning of Genome-Integrated Scaffold fused to Recruitment Domain''' | ||
| + | |||
| + | :'''Synthetic scaffold constructs fused to a leucine zipper (EE or RR) were made by cloning 500 bp of the Ste5 promoter together with the Ste5 open reading frame as a single BglII/BamHI fragment into pRS305. Zipper sequences were cloned into then cloned into BamHI/NotI sites and included a TAG stop codon immediately following their coding sequences. The Adh terminator region was cloned as NotI/SacI fragments. BglII and SacI were then used to move the synthetic scaffold cassettes into the BamHI and SacI sites of the M4366 multiple cloning site.''' | ||
| + | |||
| + | |||
| + | [[/Appendix of Sequences/]] | ||
Latest revision as of 20:40, 3 October 2007
1- Cloning Strategy
- a- Multi-part Cloning of Plasmid-Encoded Promoters, Negative Effectors and Recruitment Domains
- We have used a multipart cloning strategy based on the Type IIs restriction enzyme AarI to generate, in a single ligation, a vector containing: a promoter, a fluorescent protein tag, a negative effector, a recruitment domain (synthetic leucine zipper) and a transcription terminator. For that, we first made two families of “Donor” and “Acceptor” vectors, based on the yeast expression vector pRS315. The 5 donor vectors contain a single part (RFP tag; negative effectors YopH, YopJ or OspF; synthetic leucine zippers for recruitment, “EE” or “RR”) cloned between two AarI sites, with distinct 4-base overhangs (named A, B, C, or D, as shown in the Figure below). The 4 acceptor vectors contain a promoter (pSte5, pCyc, pAdh, or pFig; the first three are constitutive with low, medium or high expression levels respectively, the last one is induced by activation of the mating pathway), two AarI restriction sites (each one with a distinct 4-base overhang, named A and D), and a transcription terminator (red circle); as shown in the figure below:
- Each final construct was then made by ligation into a given acceptor vector, an RFP tag, followed by a negative effector and a leucine zipper, resulting in the following constructs (one example shown in the Figure):
- b- Cloning of Genome-Integrated Scaffold fused to Recruitment Domain
- Synthetic scaffold constructs fused to a leucine zipper (EE or RR) were made by cloning 500 bp of the Ste5 promoter together with the Ste5 open reading frame as a single BglII/BamHI fragment into pRS305. Zipper sequences were cloned into then cloned into BamHI/NotI sites and included a TAG stop codon immediately following their coding sequences. The Adh terminator region was cloned as NotI/SacI fragments. BglII and SacI were then used to move the synthetic scaffold cassettes into the BamHI and SacI sites of the M4366 multiple cloning site.