|
|
| Line 1: |
Line 1: |
| | {| | | {| |
| - | |[[Image:GIntro1.png]]|| text text text ahdoewhfohoejcoweijnwdcijnejknceijnfiehrfouehrojf dvjnei eh oej eo of oe oejfioejfv oefv oef voe no nvoehvoenfov enf ov efov efv | + | |[[Image:GIntro1.png]]|| This year’s UCSF iGEM team was truly a collaborative effort between several institutions. Because UCSF lacks an undergraduate program, we thought it was an excellent opportunity to extend the educational power of the IGEM by partnering up with a local high school. Five of our iGEM team members come directly from San Francisco’s Abraham Lincoln High School and are graduates of a intensive biotechnology program taught by George Cachianes and Julie Reis. Two other students, one from Palo Alto High School and one from UC Berkeley, also joined the team. Because of the unique team composition, our program focused on communicating core concepts in research science and synthetic biology in addition to actively pursuing the iGEM challenge. As a result, our summer was spent exploring synthetic biology through creative, educational, experimental and interactive angles. |
| | |- | | |- |
| - | |[[Image:GIntro2.png]]|| put in text here put in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text here | + | |[[Image:GIntro2.png]]|| Through our summer’s work, a conceptual focus resolved: we wanted to address the significance of space within cells. As synthetic biologists, we frequently focus engineering biological systems by introducing novel functionalities. Rarely do we concern ourselves with myriad effects space has on cellular behavior. As such, our team sought to address the significant of spatial localization by direction biology through synthetic assemblies and organelles. |
| | |- | | |- |
| - | |[[Image:GIntro3.png]]||put in text hereput in text hereput in text hereput in text hereput in text herevvput in text hereput in text hereput in text herevvvput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text here | + | |[[Image:GIntro3.png]]||The following statement will probably raise few, if any objections: the processes that cells carry out are complex and many. To harness energy, maintain homeostasis, or communicate with the environment, cells must execute numerous ordered, multi-step and often interrelated pathways within the cell membrane. As such, it seems like a logistical nightmare to have the enzymes, substrates and intermediates for all of these pathways freely diffusing around in the cytoplasm. This becomes even more daunting as one thinks beyond bacteria and begins thinking about the complexities of eukaryotes. |
| | |- | | |- |
| - | |[[Image:GIntro4.png]]|| put in text here put in text here put in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text here | + | |[[Image:GIntro4.png]]|| Of course, cells have evolved to avoid this potential traffic jam. What’s more, they’ve done so with a very simple solution: spatial organization. By selectively localizing and organizing particular cellular components in different regions, messages can be quickly and accurately sent, molecules can be correctly synthesized, etc. Spatial localization can be achieved through two different mechanisms: the formation of both protein complexes, which organize and regulate, and compartments, which physically isolate the interior environment from the cytoplasm. |
| | |- | | |- |
| - | |[[Image:Gintro5.png]]|| put in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text here | + | |[[Image:Gintro5.png]]||Protein complexes can specifically control the wiring of a cellular circuit by regulating both its composition and its connectivity. This simple approach can create powerful results. Frequently, proteins are organized upon scaffold proteins, where multiple, specific docking sites specifically recruit interacting partners. This both prevents cross talk while also executing multi-step tasks efficiently. |
| | |- | | |- |
| - | |[[Image:Gintro56.png]]|| put in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text here | + | |[[Image:Gintro56.png]]|| Distinct, membrane-bound compartments within a cell also offers a powerful advantage. The isolation conferred by membranes provides many benefits: Business of the interior does not interfere with business of the cytoplasm (and vice-versa). Unique environments can be created within (pH, unique concentrations). Higher local concentrations can be achieved through the benefit of a reduced volume.Controlling what’s inside and what’s outside can create specific protein-protein interactions. As a result, cells can power specific circuits, functioning in specific environments; the compartmental membrane can either protect this circuit from the outside environment, or protect the outside environment from this new circuit. |
| | |- | | |- |
| - | |[[Image:Gintro7.png]]|| put in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text hereput in text here | + | |[[Image:Gintro7.png]]|| The power of space within the cell has not yet been harnessed by synthetic biology. As such, our iGEM project approached the manipulation of spatial organization through two distinct projects. |
| | + | First, by treating the scaffold as a “molecular breadboard,” we sought to rewire a kinase signaling pathway to build new cellular circuits. |
| | |- | | |- |
| - | |[[Image:Gintro8.png]]|| put in text hereput in text hereput in text hereput in text hereput in text hereput in text here | + | |[[Image:Gintro8.png]]|| Second, and slightly more ambitious, we wanted to create a stable membrane compartment within a eukaryote: we wanted to create a new organelle (or a “synthesome,” as well call it). After this is accomplished, the options are nearly limitless for its function! Two very relevant applications are the use of the new organelle as a drug or Biofuel factory. |
| | |} | | |} |
 | This year’s UCSF iGEM team was truly a collaborative effort between several institutions. Because UCSF lacks an undergraduate program, we thought it was an excellent opportunity to extend the educational power of the IGEM by partnering up with a local high school. Five of our iGEM team members come directly from San Francisco’s Abraham Lincoln High School and are graduates of a intensive biotechnology program taught by George Cachianes and Julie Reis. Two other students, one from Palo Alto High School and one from UC Berkeley, also joined the team. Because of the unique team composition, our program focused on communicating core concepts in research science and synthetic biology in addition to actively pursuing the iGEM challenge. As a result, our summer was spent exploring synthetic biology through creative, educational, experimental and interactive angles.
|
 | Through our summer’s work, a conceptual focus resolved: we wanted to address the significance of space within cells. As synthetic biologists, we frequently focus engineering biological systems by introducing novel functionalities. Rarely do we concern ourselves with myriad effects space has on cellular behavior. As such, our team sought to address the significant of spatial localization by direction biology through synthetic assemblies and organelles.
|
 | The following statement will probably raise few, if any objections: the processes that cells carry out are complex and many. To harness energy, maintain homeostasis, or communicate with the environment, cells must execute numerous ordered, multi-step and often interrelated pathways within the cell membrane. As such, it seems like a logistical nightmare to have the enzymes, substrates and intermediates for all of these pathways freely diffusing around in the cytoplasm. This becomes even more daunting as one thinks beyond bacteria and begins thinking about the complexities of eukaryotes.
|
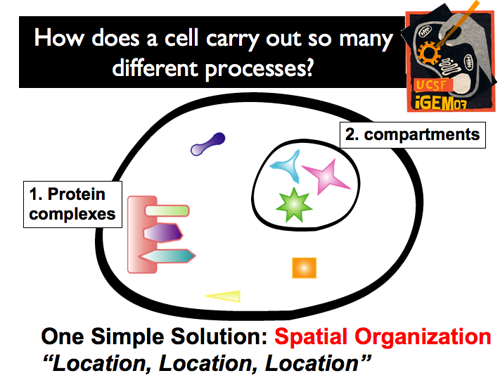 | Of course, cells have evolved to avoid this potential traffic jam. What’s more, they’ve done so with a very simple solution: spatial organization. By selectively localizing and organizing particular cellular components in different regions, messages can be quickly and accurately sent, molecules can be correctly synthesized, etc. Spatial localization can be achieved through two different mechanisms: the formation of both protein complexes, which organize and regulate, and compartments, which physically isolate the interior environment from the cytoplasm.
|
 | Protein complexes can specifically control the wiring of a cellular circuit by regulating both its composition and its connectivity. This simple approach can create powerful results. Frequently, proteins are organized upon scaffold proteins, where multiple, specific docking sites specifically recruit interacting partners. This both prevents cross talk while also executing multi-step tasks efficiently.
|
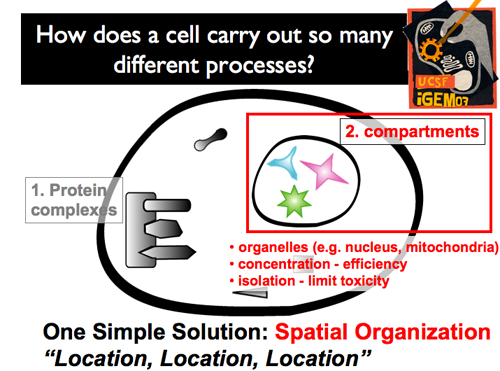 | Distinct, membrane-bound compartments within a cell also offers a powerful advantage. The isolation conferred by membranes provides many benefits: Business of the interior does not interfere with business of the cytoplasm (and vice-versa). Unique environments can be created within (pH, unique concentrations). Higher local concentrations can be achieved through the benefit of a reduced volume.Controlling what’s inside and what’s outside can create specific protein-protein interactions. As a result, cells can power specific circuits, functioning in specific environments; the compartmental membrane can either protect this circuit from the outside environment, or protect the outside environment from this new circuit.
|
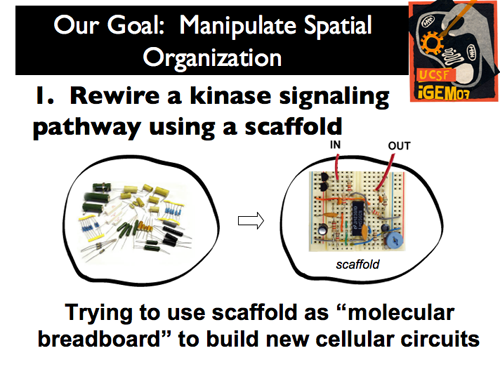 | The power of space within the cell has not yet been harnessed by synthetic biology. As such, our iGEM project approached the manipulation of spatial organization through two distinct projects.
First, by treating the scaffold as a “molecular breadboard,” we sought to rewire a kinase signaling pathway to build new cellular circuits.
|
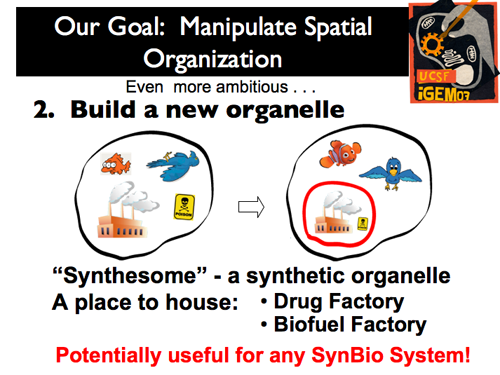 | Second, and slightly more ambitious, we wanted to create a stable membrane compartment within a eukaryote: we wanted to create a new organelle (or a “synthesome,” as well call it). After this is accomplished, the options are nearly limitless for its function! Two very relevant applications are the use of the new organelle as a drug or Biofuel factory.
|







