ETHZ/Model
From 2007.igem.org
| Line 61: | Line 61: | ||
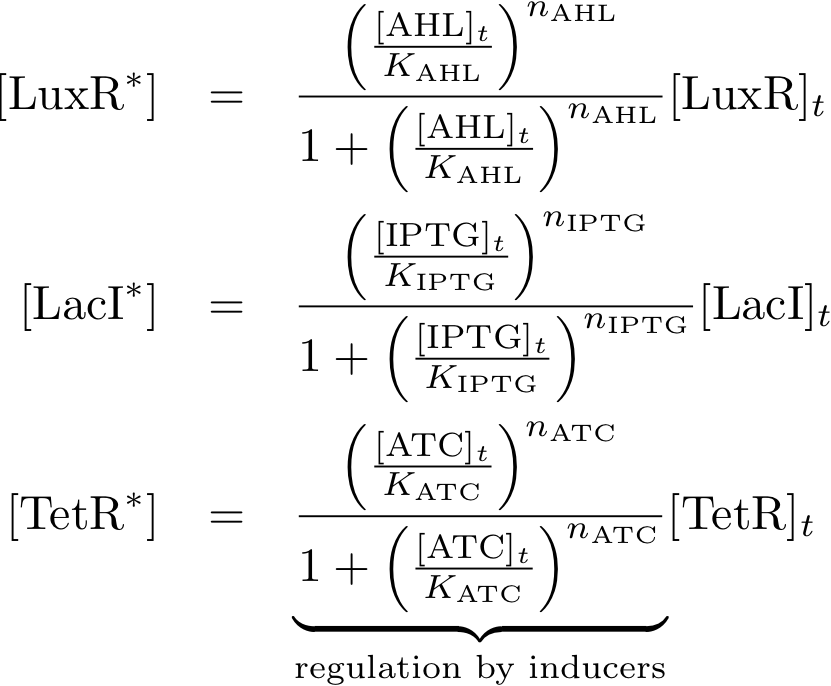
== Equations == | == Equations == | ||
| + | |||
| + | ==== Constitutively produced proteins ==== | ||
| + | |||
| + | [[Image:Eq01.png|171px]] | ||
| + | |||
| + | ==== Learning system ==== | ||
| + | |||
| + | [[Image:Eq02.png|475px]] | ||
| + | |||
| + | ==== Reporter system ==== | ||
| + | |||
| + | [[Image:Eq03.png|586px]] | ||
| + | |||
| + | ==== Allosteric regulation ==== | ||
| + | |||
| + | [[Image:Eq04.png|208px]] | ||
Revision as of 11:20, 15 October 2007
Contents |
Test Protocol
For our project we decided on designing a systems that is able to learn or adapt to its environment. Please note that this is only a minimal system that should be able to act as a proof of concept. A protocol how the system should react according to an input is shown in Figure 1.

The idea behind this protocol is that
- The system will be able to learn one of two input signals - aTc or IPTG - furing a learning phase if no input signal AHL is present. Depending on the input it will report by either green or yellow florescence.
- Once the system learned, the inputs - aTc or IPTG - can be released and the system goes into a memory state in the presence of AHL. In this state no output color is reported. Since the inputs aTc and IPTG are not present during this state we force our system to really memorize to perform the next phase properly.
- During a recognition phase the inputs aTc or IPTG are (re-) presented again. The system reports by changing its color depending on the input and its current memory state. That is why the system can have different florescence properties even in the presence of the same input. The recognition phase takes place in the presence of AHL to keep the memory enabled and avoid another learning phase.
Model Overview
To define our system we start with the classical back box approach as shown in Figure 2.
To fill that back box we have to think a bit more about the properties of our system. From our protocol we know that we need:
- 2 inputs that should be learned/detected/adapted to,
- 1 input to switch on the memory.
- We need to store at least 3 states. That is why we decided to use 2 state variables - cI and p22cII.
- We need 4 florescense signals for the outputs. Actually one could also decide to take 6 output signals into account to further distinguish the learning phase from the recognition phase but we restricted ourself to 4 outputs to reduce the number of genes that we will need to implement the signals.
An overview about the final system is shown in Figure 3.

- To be more robust against pertubations we couple the state variables cI and p22cII in the way that is well known from memory circuits that engineers build where one state variable is depressing the other one.
- We know that the system should finally be implemented in form of DNA and proteins in a bacteria. Since - due to their size - proteins can only hardly pass the cell membrane (if they are not actively transported through the cell membrane) we decided on the much smaller inducer molecules AHL, IPTG and aTc to act as the inputs. However, since those inducers cannot directly act on the transciption of DNA nor on the production of proteins we need to produce the sensor proteins LuxR, LacI and TetR that build complexes with AHL, IPTG and aTc, respectively.
- The sensor proteins and complexes are used to control the memory formation and the production of the florescence reporter proteins YFP, RFP, CFP and GFP.
Detailed Model
In this section we are transfering our model into a more detailed desciptions of the involved molecules and proteins.
Sensors
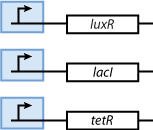
As shown in Figure 4, the proteins that act as sensors for the inducer signals are constituitively produced.
Memory
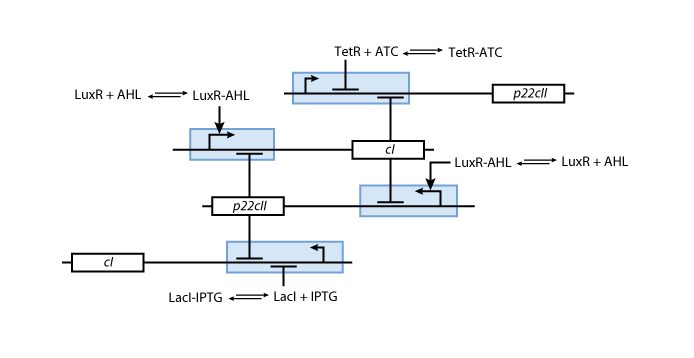
The mechanisms for the memory control are shown in Figure 5.
WE NEED BETTER FIGURE!!! SHOW PATHWAYS FOR LEARNING AND MEMORY (INNER TOGGLE SWITCH)
- The sensor proteins form complexes together with the inducers. These complexes are used to either activate (in case of the complex consisting of luxR and AHL) or repress (in case of the complexes consisting of LacI and IPTG as well as TetR and aTc) the DNA transciption of the proteins cI and p22cII.
- Futhermore, p22cII and cI repress the DNA transciption of each other.
The final mechanism that is formed is working like this:
- During the learning or training phase there is no cI or p22cII produced so far. If either IPTG or aTc is added, cI or p22cII are produced, respecively. Since no AHL is present the inner toggle switch is turned off.
- During the memory phase AHL is added and the IPTG and aTc are removed. That is why the inner toggle switch is turned off and depending on what is already present either the production of cI or p22cII is continued.
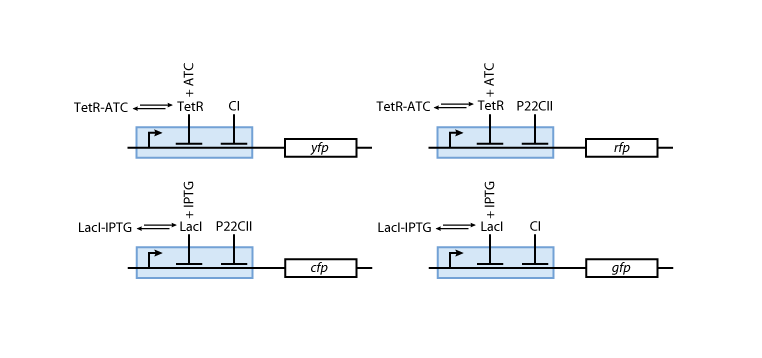
Reporters
Figure 6 gives an overview about the reporter system. Reporter proteins are expressed depending on the inducer concentrations and the concentrations of cI and p22cII.