Freiburg07/report Ca sensor
From 2007.igem.org
m |
|||
| (6 intermediate revisions not shown) | |||
| Line 5: | Line 5: | ||
Some enzymes can be divided into two separate parts that don´t show any activity until they are brought together physically.<br> | Some enzymes can be divided into two separate parts that don´t show any activity until they are brought together physically.<br> | ||
This circumstance allows enzyme-activity-assays; those assays were already being used in our lab before. We could access plasmids containing enzyme-halfs of beta-lactamase and dihydrofolatereductase (DHFR) and came up with the idea to attach those enzyme-halfs to several "trigger-proteins" which would bring them together by a mechanical movement.<br> | This circumstance allows enzyme-activity-assays; those assays were already being used in our lab before. We could access plasmids containing enzyme-halfs of beta-lactamase and dihydrofolatereductase (DHFR) and came up with the idea to attach those enzyme-halfs to several "trigger-proteins" which would bring them together by a mechanical movement.<br> | ||
| - | Cloning the DNA for each one of the enzyme-halfs and the trigger-protein together in one plasmid would provide the code for a whole "protein-machinery", allowing expression and purification as well as in vivo-tests and | + | Cloning the DNA for each one of the enzyme-halfs and the trigger-protein together in one plasmid would provide the code for a whole "protein-machinery", allowing expression and purification as well as in vivo-tests and sensoring-features of the engineered protein, an on/off-switchable enzyme.<br> |
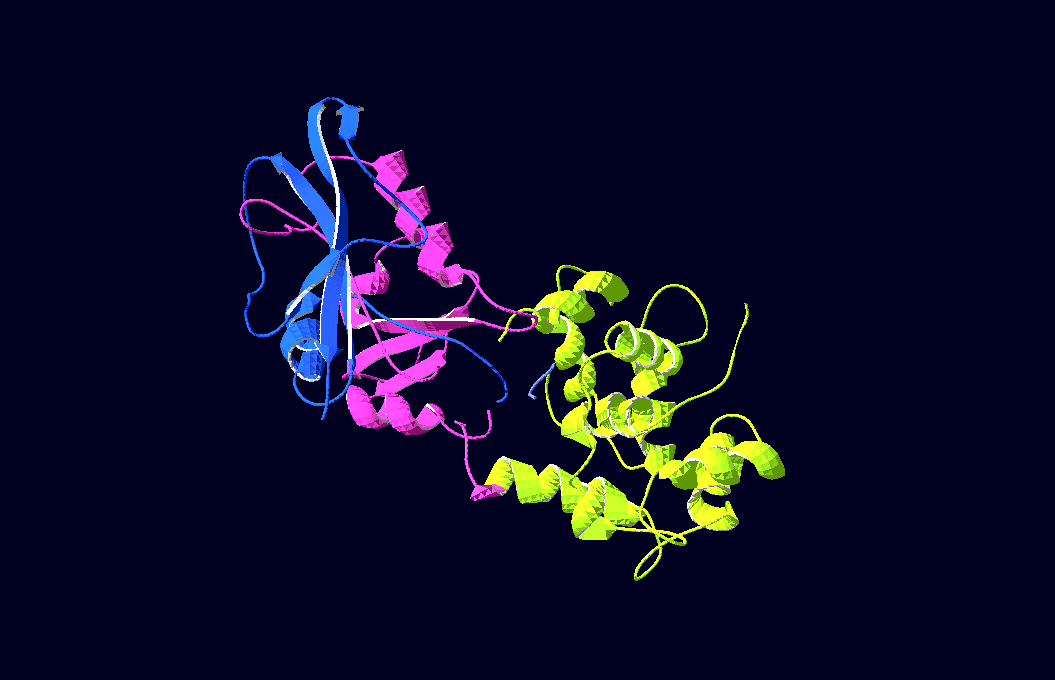
[[Image:DHFR_Calmodulin_Ca_sensor.png|thumb|398px| DHFR Calmodulin Ca2+ sensor. Image adapted from Truong et al. 2001]] | [[Image:DHFR_Calmodulin_Ca_sensor.png|thumb|398px| DHFR Calmodulin Ca2+ sensor. Image adapted from Truong et al. 2001]] | ||
| Line 11: | Line 11: | ||
Both of these enzymes suited our purpose quite well as we knew we could directly test the functionality of our constructs by simple growth tests:<br> | Both of these enzymes suited our purpose quite well as we knew we could directly test the functionality of our constructs by simple growth tests:<br> | ||
* ''E. colis carrying our plasmid would only survive if the protein-machinery was "switched on" and, thus, the enzyme "set to active"''.<br> | * ''E. colis carrying our plasmid would only survive if the protein-machinery was "switched on" and, thus, the enzyme "set to active"''.<br> | ||
| - | Now, all we needed was a protein performing a strong conformational change in reaction to an | + | Now, all we needed was a protein performing a strong conformational change in reaction to an external signal to fuse our enzyme-fragments to. And, of course, the signal should feature easy access and control.<br> |
In the beginning we had several candidates, e.g. maltose-binding-proteins, but during the research we stumbled over the calcium-sensing protein calmodulin. | In the beginning we had several candidates, e.g. maltose-binding-proteins, but during the research we stumbled over the calcium-sensing protein calmodulin. | ||
This molecule is known for its strong conformational change upon binding calcium.<br> Truong, Ikura et al. (Ontario Cancer Institute and Department of medical Biophysics, University of Toronto) have already fused CFP and YFP to the ends of a modified form of calmodulin and shown that you can then induce fluorescence resonance energy transfer (FRET) between them by adding calcium.<br> Their work inspired us to test calmodulin as "switch" for our split enzymes, especially as the fluorescent proteins have almost the same size as our enzyme-halfs. Prof. Ikura was so friendly to send us his already optimized calmodulin (YC6.1 in plasmid pcDNA3 with sequence, thanks a lot again!) so that we could gain it via PCR and start working...<br> | This molecule is known for its strong conformational change upon binding calcium.<br> Truong, Ikura et al. (Ontario Cancer Institute and Department of medical Biophysics, University of Toronto) have already fused CFP and YFP to the ends of a modified form of calmodulin and shown that you can then induce fluorescence resonance energy transfer (FRET) between them by adding calcium.<br> Their work inspired us to test calmodulin as "switch" for our split enzymes, especially as the fluorescent proteins have almost the same size as our enzyme-halfs. Prof. Ikura was so friendly to send us his already optimized calmodulin (YC6.1 in plasmid pcDNA3 with sequence, thanks a lot again!) so that we could gain it via PCR and start working...<br> | ||
| Line 36: | Line 36: | ||
'''Planning''':<br> | '''Planning''':<br> | ||
| - | -3D-Models of our constructs (as shown in the pictures above) have been created/modified using (Mac-)[http://pymol.sourceforge.net/ Pymol] and [http://expasy.org/spdbv/ SwissPdbViewer] | + | -3D-Models of our constructs (as shown in the pictures above) have been created/modified using (Mac-)[http://pymol.sourceforge.net/ Pymol] and [http://expasy.org/spdbv/ SwissPdbViewer]<br> |
-Plasmid maps, sequences, alignments and protein dates have been worked out using gcg, a unix-based, rather antiquate genetic engineering program<br> | -Plasmid maps, sequences, alignments and protein dates have been worked out using gcg, a unix-based, rather antiquate genetic engineering program<br> | ||
| Line 92: | Line 92: | ||
| - | + | '''ß-Lactamase:''' | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | At first our work was concentrated on the split ß-lactamase construct. Finally we had cloned the right plasmid, but could not go on working with this construct. We tried to amplificate E.colis with the ß-lactamase-calmodulin plasmid several times, but the bacteria carrying the right plasmid were not able to grow -the product seemed to be toxic for them: <br> | |
| - | + | *result of [https://2007.igem.org/Freiburg07/In_vivo_test_I In vivo test I]:<br> | |
| - | + | ||
| - | + | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<br> | <br> | ||
<table id="table1" border="1" width="86%"> | <table id="table1" border="1" width="86%"> | ||
| Line 156: | Line 144: | ||
</table> | </table> | ||
<br> | <br> | ||
| - | |||
On the control plates without antibiotics (Amp) the bacteria grew normally.<br> | On the control plates without antibiotics (Amp) the bacteria grew normally.<br> | ||
| + | |||
| + | <div style="float:right"> | ||
| + | {| | ||
| + | |- | ||
| + | | class="taxo-image" | [[Image:growth3.jpg|thumb|398px|'''Picture:''' Growth of E.coli with DHFR1-4Gly-Calmo-4Gly-DHFR2A in minimal medium at different Ca2+-concentrations]] | ||
| + | |}</div> | ||
| + | |||
| + | |||
| + | |||
| + | '''DHFR:''' | ||
| + | |||
| + | In-Vivo-Analysis:<br> | ||
| + | As DHFR is essential for bacterial growth in minimal medium with trimethioprim (TMP), | ||
| + | we could show that the ability of E.colis with our plasmid to grow is directly dependent on the presence -and, furthermore, concentration- of calcium-ions (Ca2+)in the medium([https://2007.igem.org/Freiburg07/In_vivo_test_II In vivo test II]):<br>In this test we inoculated several probes of M9 minimal medium with different calcium concentrations with DHFR1-4Calmodulin4-DHFR2A-positive E. colis and measured their optical densities (example: first measurement after 24 hours and then again every three to 10 hours for another two days). Several tests we performed before made this approach seem best for finding out about the relation between Ca2+-concentration and cell survival; unfortunately we ran out of time so that we couldn´t quantify our results the way we would have liked to.<br> | ||
| + | |||
'''Plasmids:''' | '''Plasmids:''' | ||
Latest revision as of 00:04, 27 October 2007
Contents |
A single-protein calcium ion sensor mediating cell survival
iGEM team Freiburg
Introduction
Some enzymes can be divided into two separate parts that don´t show any activity until they are brought together physically.
This circumstance allows enzyme-activity-assays; those assays were already being used in our lab before. We could access plasmids containing enzyme-halfs of beta-lactamase and dihydrofolatereductase (DHFR) and came up with the idea to attach those enzyme-halfs to several "trigger-proteins" which would bring them together by a mechanical movement.
Cloning the DNA for each one of the enzyme-halfs and the trigger-protein together in one plasmid would provide the code for a whole "protein-machinery", allowing expression and purification as well as in vivo-tests and sensoring-features of the engineered protein, an on/off-switchable enzyme.
Both of these enzymes suited our purpose quite well as we knew we could directly test the functionality of our constructs by simple growth tests:
- E. colis carrying our plasmid would only survive if the protein-machinery was "switched on" and, thus, the enzyme "set to active".
Now, all we needed was a protein performing a strong conformational change in reaction to an external signal to fuse our enzyme-fragments to. And, of course, the signal should feature easy access and control.
In the beginning we had several candidates, e.g. maltose-binding-proteins, but during the research we stumbled over the calcium-sensing protein calmodulin.
This molecule is known for its strong conformational change upon binding calcium.
Truong, Ikura et al. (Ontario Cancer Institute and Department of medical Biophysics, University of Toronto) have already fused CFP and YFP to the ends of a modified form of calmodulin and shown that you can then induce fluorescence resonance energy transfer (FRET) between them by adding calcium.
Their work inspired us to test calmodulin as "switch" for our split enzymes, especially as the fluorescent proteins have almost the same size as our enzyme-halfs. Prof. Ikura was so friendly to send us his already optimized calmodulin (YC6.1 in plasmid pcDNA3 with sequence, thanks a lot again!) so that we could gain it via PCR and start working...
Materials and Methods
We had practically full access to a well-equipped genetic engineering laboratory at our university.
Planning:
-3D-Models of our constructs (as shown in the pictures above) have been created/modified using (Mac-)[http://pymol.sourceforge.net/ Pymol] and [http://expasy.org/spdbv/ SwissPdbViewer]
-Plasmid maps, sequences, alignments and protein dates have been worked out using gcg, a unix-based, rather antiquate genetic engineering program
Protein expression and purification:
-Expression cultures (Volume: 2 liters) have been raised at 37 deg. C, induced by IPTG and harvested at optical densities around 2, as they usually had reached their stationary phase by then
-Protein purifications have been carried out via Ni-NTA-columns; the His-Tagged proteins have been eluted in buffers with rising imidazole-concentrations and were then analyzed on SDS-gels (e. g. below):
Cloning:
-Cloning steps have been done as described below:
Mediums and Plates
DNA sequencing
Ligation
Plasmid spin column prep
Glycerol stocks
General Gene-Protein Information
Purification
Transformation
Dephosphorylation
Preparative Digestion
Analytic Digestion
Gel Electrophoresis
Polyacrylamide gel electrophoresis
Protein purification
In vivo test I
In vivo test II
Calmodulin via PCR:
forward primers:
pf-calmo-KpnI-linker2: 5' TATCGACGGT ACCGGCGGTG AGCAGATTGC AGAGTTCAAA G 3'
pf-calmo-KpnI-linker3: 5' TATCGACGGT ACCGGCGGTG GCGAGCAGAT TGCAGAGTTC AAAG 3'
pf-calmo-KpnI-linker4: 5' TATCGACGGT ACCGGCGGTG GCGGTGAGCA GATTGCAGAG TTCAAAG 3'
pf-calmo-KpnI-linker6: 5' TATCGACGGT ACCGGCGGTG GCTCTGGTGG CGAGCAGATT GCAGAGTTCA AAG 3'
pf-calmo-KpnI-linker9: 5' TATCGACGGT ACCGGCGGTG GCGGTTCTGG CGGTGGCGGT GAGCAGATTG CAGAGTTCAA AG 3'
reverse primers:
pr-calmo-SpeI-linker2: 5' AACGATCACT AGTACCTCCC TTTGCTGTCA TCATTTGTAC A 3'
pr-calmo-SpeI-linker3: 5' AACGATCACT AGTACCGCCA CCGCCCTTTG CTGTCATCAT TTGTACA 3'
pr-calmo-SpeI-linker4: 5' AACGATCACT AGTGCCACCG CCTCCCTTTG CTGTCATCAT TTGTACA 3'
pr-calmo-SpeI-linker6: 5' AACGATCACT AGTACCGCCA CCAGAGCCAC CCTTTGCTGT CATCATTTGT ACA 3'
pr-calmo-SpeI-linker9: 5' AACGATCACT AGTACCGCCA CCGCCAGAAC CGCCACCGCC CTTTGCTGTC ATCATTTGTA CA 3'
Results
ß-Lactamase:
At first our work was concentrated on the split ß-lactamase construct. Finally we had cloned the right plasmid, but could not go on working with this construct. We tried to amplificate E.colis with the ß-lactamase-calmodulin plasmid several times, but the bacteria carrying the right plasmid were not able to grow -the product seemed to be toxic for them:
- result of In vivo test I:
| Ca2+ [µg/ml] | 0 | 0.1 | 1.0 | 10 |
| IPTG [M] | 1 | 1 | 1 | 1 |
| Amp [µg/ml] | 50 | 50 | 50 | 50 |
| 2.2 Gly-linker calmodulin [colonies] | 0 | 0 | 0 | 0 |
| 4.4 Gly-linker calmodulin [colonies] | 0 | 0 | 0 | 0 |
| 6.6 Gly-linker calmodulin [colonies] | 0 | 0 | 0 | 0 |
On the control plates without antibiotics (Amp) the bacteria grew normally.
DHFR:
In-Vivo-Analysis:
As DHFR is essential for bacterial growth in minimal medium with trimethioprim (TMP),
we could show that the ability of E.colis with our plasmid to grow is directly dependent on the presence -and, furthermore, concentration- of calcium-ions (Ca2+)in the medium(In vivo test II):
In this test we inoculated several probes of M9 minimal medium with different calcium concentrations with DHFR1-4Calmodulin4-DHFR2A-positive E. colis and measured their optical densities (example: first measurement after 24 hours and then again every three to 10 hours for another two days). Several tests we performed before made this approach seem best for finding out about the relation between Ca2+-concentration and cell survival; unfortunately we ran out of time so that we couldn´t quantify our results the way we would have liked to.
Plasmids:
We gained plasmids containing both parts of each split-enzyme, linked to calmoduline by 2/4/6 glycins each;
in-vivo-growth tests with DHFR construct showed the best activity for 4-glycine linkers;
thus we decided to proceed with this linker-length.
Ca2+ sensor: ß-lactamase
Plasmids with calmodulin between the lactamase fragments:
2 amino acids for linker: pFR320p_b1_2calmo2_b2
4 amino acids for linker: pFR320p_b1_4calmo4_b2
6 amino acids for linker: pFR320p_b1_6calmo6_b2
Ca2+ sensor: DHFR
Plasmids with calmodulin between the dhfr fragments:
2 amino acids for linker: dFR320d-dhfr1-2calmo2-dhfr2A
4 amino acids for linker: dFR320d-dhfr1-4calmo4-dhfr2A
6 amino acids for linker: dFR320d-dhfr1-6calmo6-dhfr2A
Discussion
Our goal to create a fusion-protein sensor with functional nano-mechanical aspects seems to be achieved at least for DHFR.
Cloning the beta-lactamase plasmid as planned, we encountered various problems such as unexpected mutations and the circumstance that the product seemed to be toxic for E. coli. For this reason we designed optimized split-lactamase parts to enhance stability. Unfortunately there was no time left to prove the activity and functionality of this construct.
But we are virtually certain that this ß-lactamase-calmodulin construct without any mutation on the lactamase part is able to be regulated by Ca2+ addition.
[http://partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2007&group=Freiburg Parts] submitted
- BBa_I757003: xCaM_rCKK_xCaM_fusionpart, calmodulin
- BBa_I757005: DHFR1_1-107_FusionPart, split-DHFR-fragment no.1
- BBa_I757006: DHFR2A_109-187_FusionPart, split-DHFR-fragment no.2
- BBa_I757010: b-lactamase TEM-1 optimized, the enzyme beta-lactamase as a whole
- BBa_I757011: bla_frag1, split-beta-lactamase fragment no.1
- BBa_I757012: bla_frag2 200-263, split-beta-lactamase fragment no.2
- BBa_I757013: His_tag, a poly-histidine tag for protein purification
- BBa_I757014: StrepTagII Fusion Part,a streptavidin tag for protein purification
- All of these parts feature our optimized restriction sites for building fusion-proteins.
References
split DHFR:
Pelletier JN, Arndt KM, Plückthun A, Michnick SW. "An in vivo library-versus-library selection of optimized protein-protein interactions." Nat Biotechnol. 1999 Jul;17(7):683-90.
Pelletier JN, Campbell-Valois FX, Michnick SW. "Oligomerization domain-directed reassembly of active dihydrofolate reductase from rationally designed fragments." Proc Natl Acad Sci U S A. 1998 Oct 13;95(21):12141-6. [http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=9770453&ordinalpos=5&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum pubmed]
Calmodulin Sensor:
Pham E, Chiang J, Li I, Shum W, Truong K "A computational tool for designing FRET protein biosensors by rigid-body sampling of their conformational space." Structure. 2007 May;15(5):515-23.
Truong K, Sawano A, Miyawaki A, Ikura M. "Calcium indicators based on calmodulin-fluorescent protein fusions." Methods Mol Biol. 2007;352:71-82.
Truong K, Sawano A, Mizuno H, Hama H, Tong KI, Mal TK, Miyawaki A, Ikura M. "FRET-based in vivo Ca2+ imaging by a new calmodulin-GFP fusion molecule." Nat Struct Biol. 2001 Dec;8(12):1069-73.
Reviews on calmodulin: Grabarek Z "Structural basis for diversity of the EF-hand calcium-binding proteins." J Mol Biol. 2006 Jun 9;359(3):509-25.
Soderling TR, Stull JT "Structure and regulation of calcium/calmodulin-dependent protein kinases." Chem Rev. 2001 Aug;101(8):2341-52