Boston University/Mutation of GTFs
From 2007.igem.org
| Line 1: | Line 1: | ||
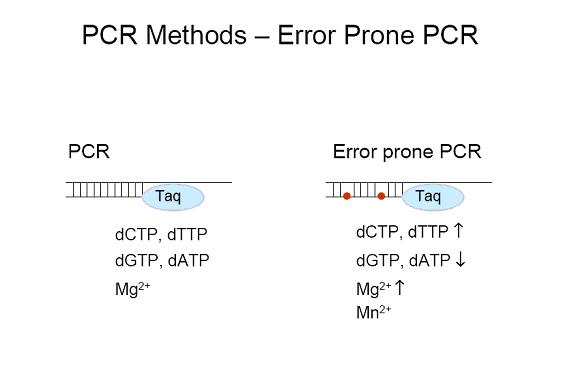
To induce favorable mutations we plan to employ directed mutagenesis on the aforementioned global transcription factors to increase current output. The mutations would be introduced by error prone PCR, which is a technique used to generate randomized genomic libraries. Error-prone PCR will allow us to initiate DNA amplification starting with tiny amounts of parent molecule to produce considerable amounts of the mutated gene. This technique is based on the principle that Taq polymerase is capable of annealing incompatible base-pairs to each other during amplification under imperfect PCR conditions. The figure below contrasts the external conditions between PCR and error prone PCR. | To induce favorable mutations we plan to employ directed mutagenesis on the aforementioned global transcription factors to increase current output. The mutations would be introduced by error prone PCR, which is a technique used to generate randomized genomic libraries. Error-prone PCR will allow us to initiate DNA amplification starting with tiny amounts of parent molecule to produce considerable amounts of the mutated gene. This technique is based on the principle that Taq polymerase is capable of annealing incompatible base-pairs to each other during amplification under imperfect PCR conditions. The figure below contrasts the external conditions between PCR and error prone PCR. | ||
| - | [[Image: | + | [[Image:BU_Error_Prone.jpg|center]] |
After the implementation of error prone PCR the mutant gene is in abundant concentration and thus has an extremely high likelihood of ligating into a viable plasmid. These random matching “errors” in the global transcription factors will presumably lead to desirable mutations ultimately causing increase in current output. Following the ligation step, the random variants will be screened and selected using ferrozine. On the other hand, the original strand can be sorted out and eliminated by enzymatic digestion, leaving behind the PCR product of the mutated gene. | After the implementation of error prone PCR the mutant gene is in abundant concentration and thus has an extremely high likelihood of ligating into a viable plasmid. These random matching “errors” in the global transcription factors will presumably lead to desirable mutations ultimately causing increase in current output. Following the ligation step, the random variants will be screened and selected using ferrozine. On the other hand, the original strand can be sorted out and eliminated by enzymatic digestion, leaving behind the PCR product of the mutated gene. | ||
Latest revision as of 16:20, 9 July 2007
To induce favorable mutations we plan to employ directed mutagenesis on the aforementioned global transcription factors to increase current output. The mutations would be introduced by error prone PCR, which is a technique used to generate randomized genomic libraries. Error-prone PCR will allow us to initiate DNA amplification starting with tiny amounts of parent molecule to produce considerable amounts of the mutated gene. This technique is based on the principle that Taq polymerase is capable of annealing incompatible base-pairs to each other during amplification under imperfect PCR conditions. The figure below contrasts the external conditions between PCR and error prone PCR.
After the implementation of error prone PCR the mutant gene is in abundant concentration and thus has an extremely high likelihood of ligating into a viable plasmid. These random matching “errors” in the global transcription factors will presumably lead to desirable mutations ultimately causing increase in current output. Following the ligation step, the random variants will be screened and selected using ferrozine. On the other hand, the original strand can be sorted out and eliminated by enzymatic digestion, leaving behind the PCR product of the mutated gene.