Chiba/Sandbox/Home2
From 2007.igem.org
|
Introduction | Project Design ( 1.Affinity Tag | 2.Communication Module | 3.Size Control ) | Making Marimos | Our Goal |
Project Overview
Our iGEM project is to make a Marimo-ish gathering of bacteria. Marimo is a green spherical shaped algae (shown in Fig.1), which is a popular living organism in Japan as a National Treasure because of its beautiful shape and its smoothness.Why We Make a Marimo : Spherical Multicellular Organism!?
When you see a shape in Nature, you will notice whether a sphere, which is absolutely symmetric in 3D, is really stable or not in Nature.
In fact, an oil droplet is a sphere in water. Red blood cell in a hypotonic solution shows its shape change to a spherical balloon. However multicellular organisms have their shape different from a sphere except Marimo. Of course, other algae do not show spherical shape, they live on a surface of stone. It is quite intriguing how Marimo remains its spherical shape in a lake!
Our team focuses understanding how such spherical structure can be sustained even when it Is multicellular organisms.
more...
How To Make Our Marimo
What we require to our system is as follows:- Affinity Tag.
- Communication Module.
- Size Control.
Two cells are used in our system: AHL senders and receivers. Senders generates the affinity tags constitutively, while receivers generates them only when they are induced by AHL. The marimo-forming goes like this:
- Make the sender core by sticking with the affinity tag.
- Insert the sender core into the receiver culture.
- The sender core produces AHL, which make the near receivers to generate the affinity tags and GFPs.
- The affinity tagged receiver sticks with the central sender core. This will continue until the AHL cannnot reach the marimo boundary,
- When the AHL reached the marimo boundary, the adsorping stops, which makes a finite-sized marimo bacteria.
Experiments Overview
Making Affinity Tags
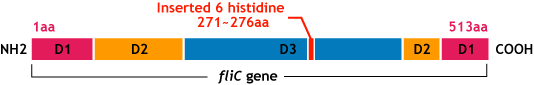
- Make the affinity tag by inserting the his-tag into the flagellar fillament.
Making Communication Module
- Making Receivers
- Making Senders
Controlling Size
- Improve Sender
- Improve Receiver
- Localize AHL
Making Marimo
Our Goal
Below we describe the goal to make a marimo bacteria.
- A test of adsorption between flagella
- A test to confirm a limit of size
- A test to form Marimo
- A test of size control
Advanced Goal in Our Future
See below link: our brainstorming of the marimo future!