Alberta
From 2007.igem.org

The Offical Wiki of the 2007 University of Alberta iGEM Team
Contents |
Background: Biofuels
With growing concerns of global greenhouse gasses, the global energy market is in a continual push for the development of renewable energy sources. Specifically, two fuels have dominated the media: biodiesel and ethanol. However, both of these fuels have shortcomings in terms of being a viable fuel source.
Biodiesel is a fuel produced from the vegetable oils of crops that can be used in an engine system very similar to a traditional diesel engine. However, vegetable oils in crops only make up a small portion of the biomass of the plants, ultimately producing low yields of fuel per acre of crops. As such, it is more economically sound to use these resources for food production.
There has been huge attention to the use of ethanol in the typical auto cycle engine. In many places it is already being blended with gasoline to create a hybrid fuel source. However, running an engine on pure ethanol is beset by several major obstacles. Firstly, ethanol is miscible with water at any concentration, which creates long term storage corrosion issues. Secondly, ethanol engines must be designed to expect water vapor unlike their gasoline counterparts. Ethanol also has significantly different thermodynamic properties than gasoline such as a lower energy density and different vapor properties, which would reduce the economical advantage of using ethanol as a primary fuels source.
We propose using a different fuel source to eventually replace gasoline, butanol. Butanol is a superior to ethanol as a replacement for petroleum gasoline. With a low vapor pressure, high energy density, and a gasoline-like octane rating, it can be blended into existing gasoline at much higher proportions than ethanol without compromising performance, mileage, organic pollution standards. Blending butanol with gasoline also prevents major modifications of the fuel-air ratio and modifications to the fuel system. Butanol also is immiscible with water at concentrations higher than 7%, alleviating storage concerns as well as offering the possiblity of phase separation which would realize huge cost savings in terms of production.
More information on the summary of biofuels viability and the inspiration to our project can be found here.
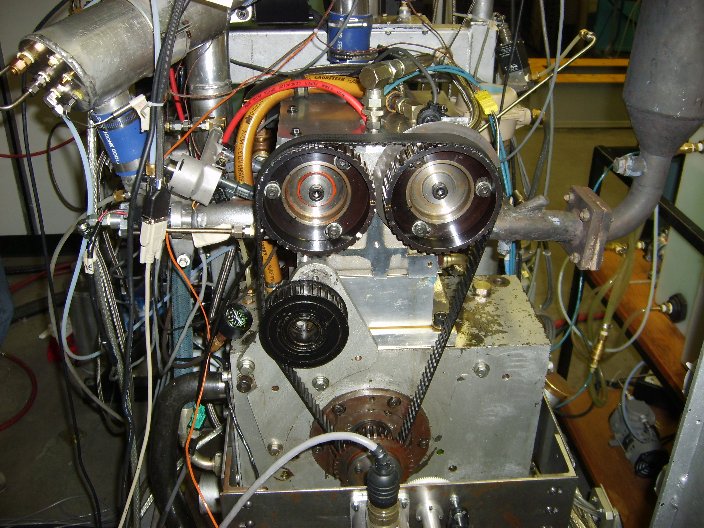
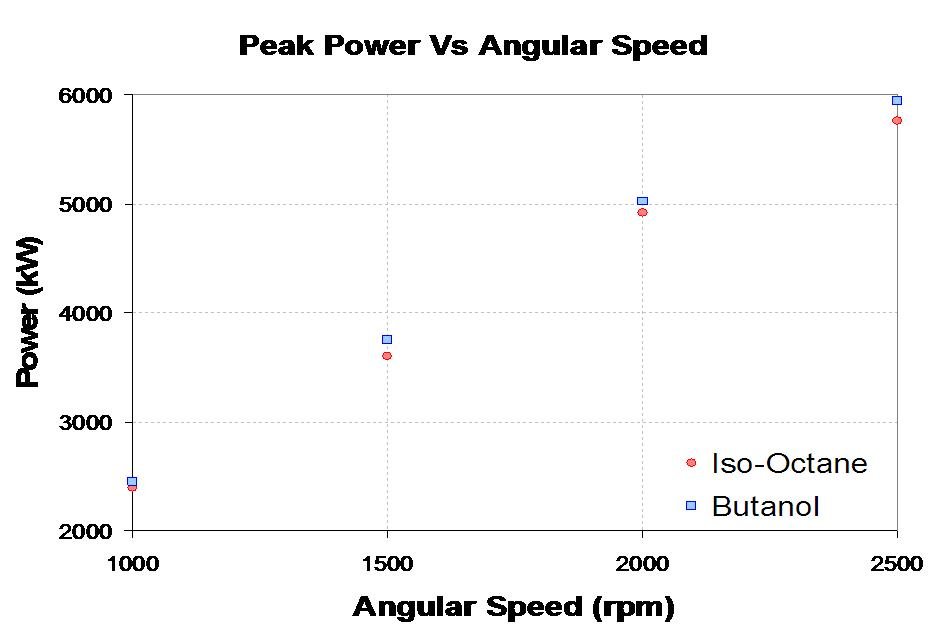
The Butanerds wanted to further evaluate the use of butanol as a fuel in standard spark ignition engines. With the assistance of The University of Alberta's Engine Control Lab in Mechanical Engineering we were able to run a peak power test comparing butanol to iso-octane, a standard test measurement. Each fuel was burned at a stoichiometric air to fuel ratio at 4 different angular speeds. The experimental setup and results can be seen below.
The Project: Plan B
We propose the use of butanol as the leading biofuel for use in internal combustion engines. Specifically, we intend to genetically engineer Escherichia coli bacteria to convert biomass into butanol for use as an energy source. This will be accomplished by introducing the genes responsible for butanol production from Clostridium acetobutylicum (i.e. endogenous butanoate pathway) into E. coli. Furthermore, we hope to increase E. coli's tolerance to solvents such as butanol.
I: Transforming genes in C. acetobutylicum's butanoate pathway into E.coli
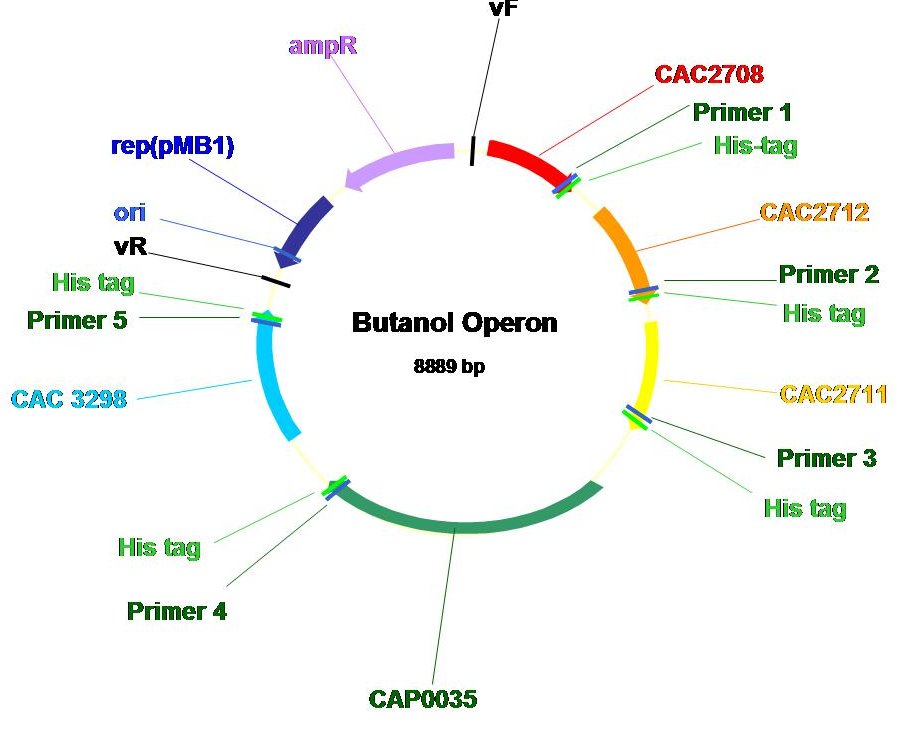
Genes encoding the enzymes in the C. acetobutylicum butanoate metabolism pathway were identified using the KEGG database (KEGG number-ca00650: http://www.kegg.com/dbget-bin/www_bget?path:cac00650). Our cloning stragegy is to incorporate all the genes in a single operon with respective inducible promoter and ribosome binding site in a plasmid. Such construct enables easy transformation of multiple genes simultaneously into E.coli and allows the coordinated expression of genes within the operon.
After receiving the commercially synthesized coding sequences of the genes of interest, the coding sequences are restricted with proper BioBrick prefixes and suffixes out of the original plasmid and cloned into B0034 (ribosome binding site). Double digest (Xba I and Spe I) as well as automated sequencing reactions were performed to verify the proper insertion of the coding sequences and the presence of a ribosome binding site at the 5' end of each coding sequence.
The operon, shown below, consists of all of the genes in the pathway (except E. coli'sthiolase). Note that we purposedly added a Histamine tag to the carboxy terminus of each gene product such that we could use Nickel-NTA column to purify the individual proteins and analyze protein expression and activity.
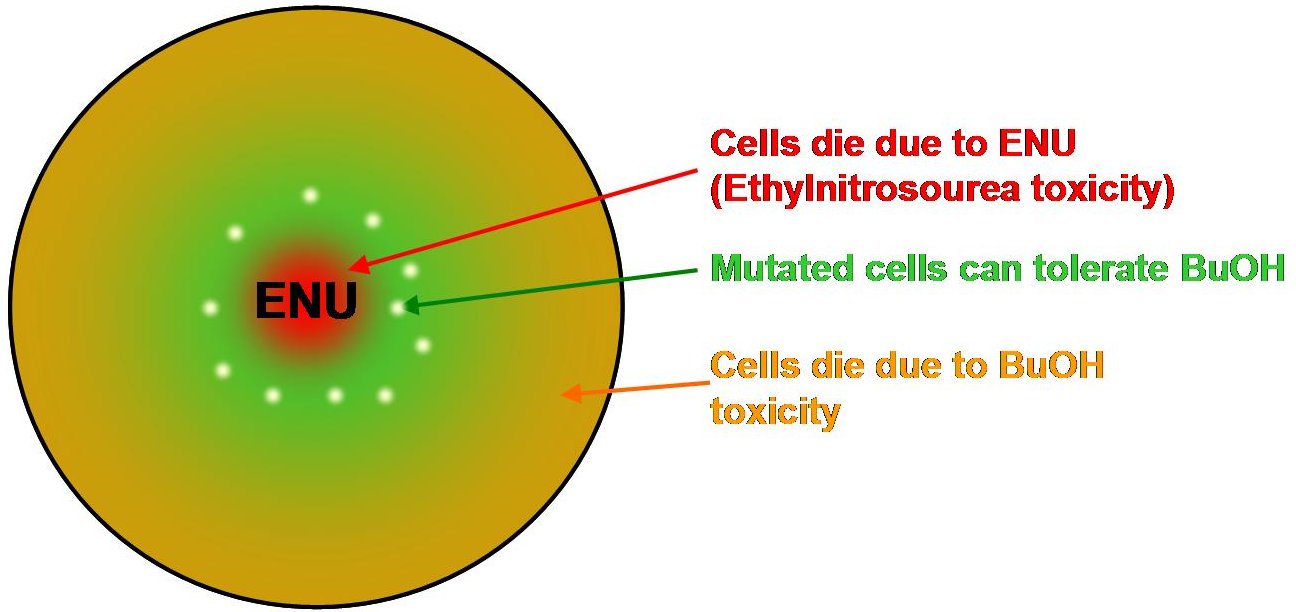
Objective 2: Develop Butanol Tolerance with mutagenic and toxic compound Ethylnitrosourea ENU.
The concept is to make butanol agar plates of various butanol concentrations and place an ENU disk in the centre. We expect there will be two kill zones. The first where bacteria die due to the toxicity of ENU at the centre of the plate. The second around the outer ring of the butanol plate where bacteria die due to butanol toxicity. In between these two zones we hope to see some cells that would be mutated in a benifical way to allow them to survive in the butanol.
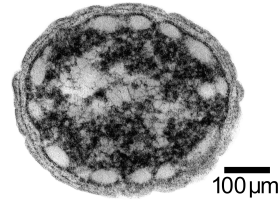
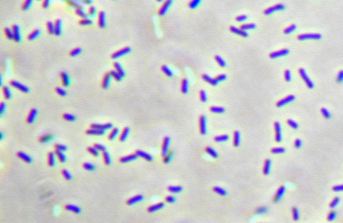
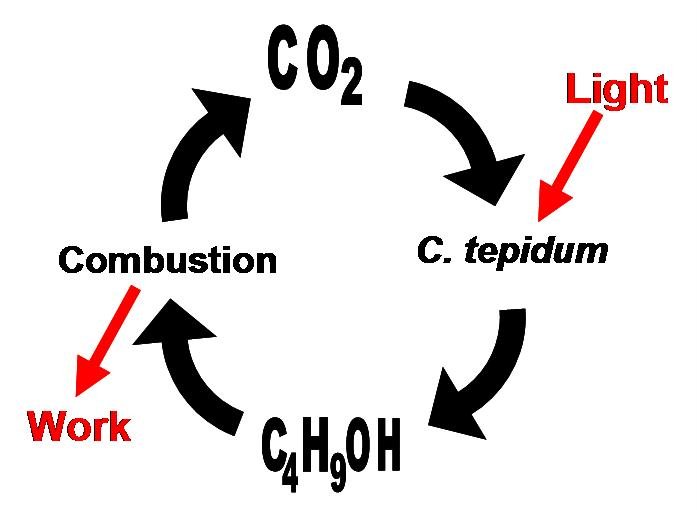
Concurrently, we are investigating the use of a photoautotrophic bacterium, Chlorobium tepidum that we will also introduce butanol producing genes to. Chlorobium tepidum is a green sulfur bacterium that is strictly anaerobic and uses sulfur compounds as terminal electron donors. Chlorobium tepidum is a moderately thermophillic bacterium, growing at 40 degrees Celsius (104 degrees Fahrenheit), and requires low light conditions for optimal growth. These bacteria grow well in a defined medium, utilizing the reverse/reductive tricarboxylic acid (TCA) cycle to build up carbohydrates from carbon dioxide. For a more comprehensive overview of Chlorobium tepidum go to [[http://www.bmb.psu.edu/faculty/bryant/lab/chlorobiumtepidum/index.html here]]. Images below from http://www.bmb.psu.edu/faculty/bryant/lab/chlorobiumtepidum/index.html
The advantage of manipulating this organism for butanol production is a matter of energy input and output. Rather than having to utilize vast amounts of food stocks, such as grains or sugars, a photoautotroph will fix carbon dioxide into the complex carbohydrates required for butanol production. Theoretically, if the only fuel on our planet was butanol, and it was produced in this manner, there would be little net carbon dioxide produced.
Modeling Efforts
Initially our plan to model the efficiency of the butanol production hinged on developing a complete model for the entire glucose pathway in an E. coli cell using Michaelis-Menten kinetics. After further review, this proved to be beyond the scope of our project's timeline due both to the large number of species and reactions present in the system (as well as their non-linear behavior) and the relative difficulty in finding experimental kinetic information for some of the reactions in our pathway.
Therefore, to obtain a rough "first-order" approximation for the behavior of the pathway, we considered three factors:
1) Stoichiometric factors (both redox and carbon)
2) Compared the relative production rates of the various end products of the glucose pathway in E. coli as found in previous experimentation to the production rates of butanol in C. acetobutylicum.
3) We have also a fuel E. coli Stoichometric Matrix, and intend to stoichiometrically model the system in order to optimize butanol production once the operon is in the system.
From these factors we can obtain an indicator of how likely butanol will be produced.
In order to further develop the system, we hope a full metabolic stochiometric model can be eventually realized for this project. This would allow optimization of the system in order to maximize the flux through the butanol pathway.
Project Timeline
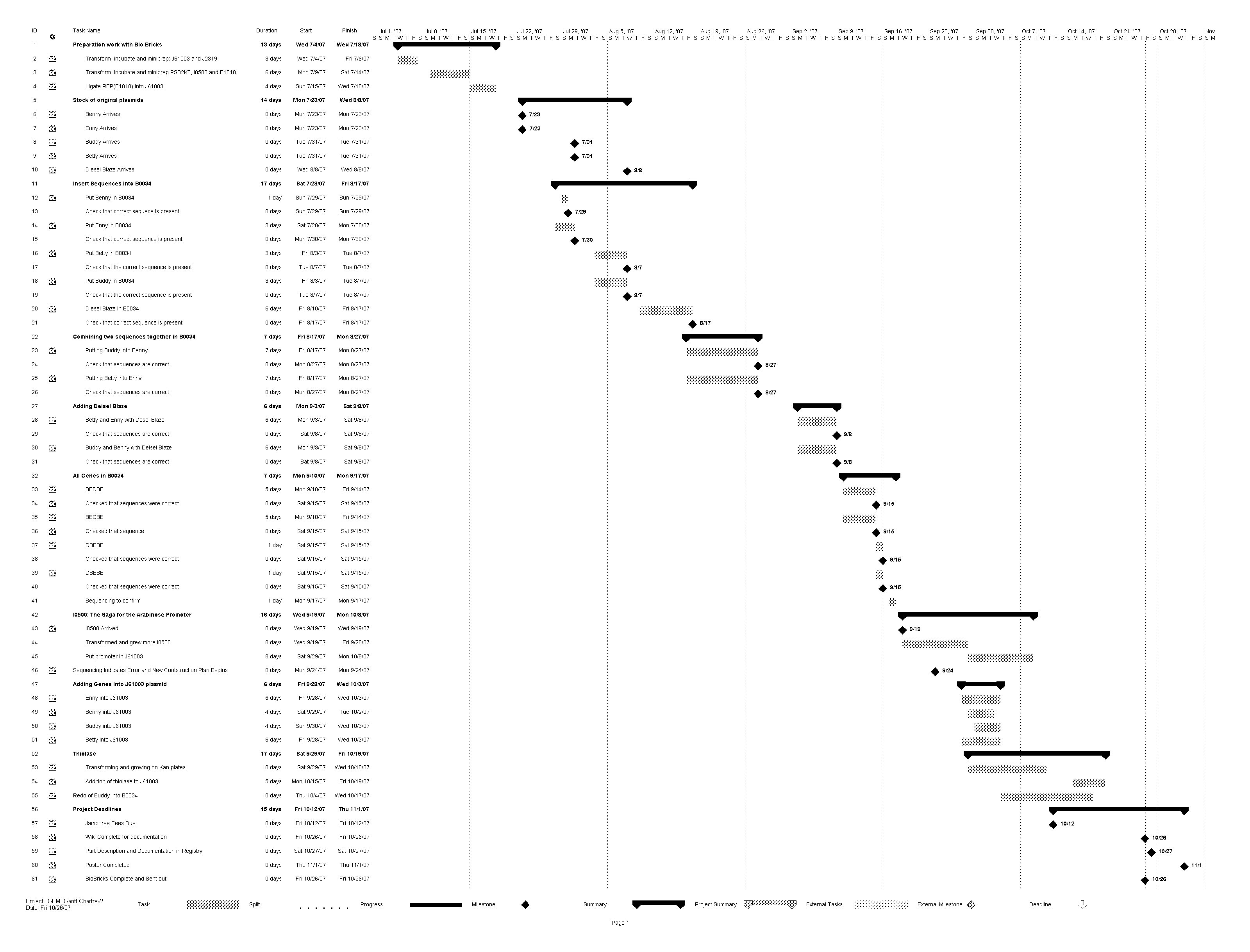
The GANTT Chart Above details the schedule for the lab work that contributed to Plan B. Beginning in July and finishing with our final work in October, the schedule includes details on our different methods to compose an operon that would meet our objectives. In order to read the GANTT Chart for the Lab work of Plan B, please refer to the legend below.
Legend:
Benny - Butyryl CoA dehydrogenase
Enny - Enoyl CoA hydratase
Buddy - Butanol dehydrogenase
Betty - Beta-hydraoxy butyryl CoA dehydrogenase
Deisel Blaze - Butyraldehyde dehydrogenase
Lab Book and Calendar
Butanerd Event Calendar can be found below:
[http://www.ualberta.ca/~mjl3/UofAIgemProtocols.pdf The Lab Protocols]
[http://butanerds.myfreeforum.org The Butanerd Online Forum] is up and running. Feel free to post and check for posts here. Make sure you register!
The Team

Our team has a rich background in biology, biochemistry and engineering. To compliment our diversity we also have advisors who have a wealth of knowledge in research and applications of genetic engineering. For more information about the group, check out the University of Alberta's iGEM Team Members Page.
Our University of Alberta Student Group Constitution can be found here
Edmonton
Welcome to Edmonton!

For more on the city of Edmonton click [http://www.ualberta.ca/~mjl3/About.html here].
Sponsors
We would like to thank all of our sponsers for their gracious support and our advisors for invaluble advise.
Major Sponsors

|
Sponsors

|
Advice
Andrew Hessel - Alberta Ingenuity Mentor
Dr. Jonathan Dennis - University of Alberta
Dr. Perrin Beatty - University of Alberta
Dr. David Bressler - University of Alberta
Dr. Charles Lucy - University of Alberta
Dr. Gregory Kiema - University of Alberta
Dr. Mark S. Peppler - University of Alberta
Dr. James Harynuk - University of Alberta
Dr. Federick West-University of Alberta
Dr. Todd Lowary-University of Alberta
Desiree Schell - University of Alberta CJSR
Dr. Jeff Fuller - University of Alberta/Capital Health
Dr. Julia Foght - University of Alberta
Dr. David Checkel - University of Alberta
Adrian Audiet - University of Alberta
Dr. Koch - University of Alberta
Dr. Donald Bryant - Pennsylvania State
Dr. Gaozhong Shen - Pennsylvania State
Amaya Garcia - Pennsylvania State
A list of experimental supplies we need can be found here
External Links
[http://www.albertaingenuity.ca/ Alberta Ingenuity Fund]
[http://www.ualberta.ca The University of Alberta Hompage]
[http://www.mece.ualberta.ca/~ckoch/ Dr. Koch's Engine Lab]
[http://www.systems-biology.org/cd Cell Designer Homepage]
[http://www.biomodels.org Biomodels Home Page]