Arthur Yu Notebook
From 2007.igem.org
| Line 3: | Line 3: | ||
[[Template:BerkiGEM2007_ArthurSequencingFiles | My Sequencing Files]]<br><br> | [[Template:BerkiGEM2007_ArthurSequencingFiles | My Sequencing Files]]<br><br> | ||
---- | ---- | ||
| + | ==7/17 yaeyeayea == | ||
| + | * iron stuff growing tubes. 16 tubes, 4+4 clones jp style and not | ||
| + | * 12 and 19 are growing plate. | ||
==7/16 four ligations== | ==7/16 four ligations== | ||
* the sequencing for wbbL/neuS library seems like Ptet is cut out. bleh doing again | * the sequencing for wbbL/neuS library seems like Ptet is cut out. bleh doing again | ||
Revision as of 23:36, 17 July 2007
My Construction Files
My Sequencing Files
7/17 yaeyeayea
- iron stuff growing tubes. 16 tubes, 4+4 clones jp style and not
- 12 and 19 are growing plate.
7/16 four ligations
- the sequencing for wbbL/neuS library seems like Ptet is cut out. bleh doing again
- made I716008, I716018, I716017, I716016 and plated
7/13 friday
- the RFP without ATG looks good (sequencing)
- wbbl/neuS lib didn't work again hmm, sent for sequencing
- other stuff will insert later
7/12 efficiency
- got news that I716012 didn't sequence well, trying amplify
- incubated I716015 (pBca9145-RFP_noATG)
- miniprepped yfbE's (I716013 and I716014)
- plated proprietary I716016 on FeCl3 and without
- new yfbE stuff for sequencing
- running gel on I716015 pcr prod to expediate testing
- redo I716012 again
- digesting 101 and 008
- growing up electrocompetant MC828E
remarks austin said cytochrome b5 /b5 red was pretty. yeayeayeayaeyea
7/11 seven eleven
- miniprep'd wbbL/neuS funny thing
- restriction map looks funny (see right)
- sent it for sequencing.
- poured super special david plates
- basic parted RFP without start ATG, and plated.
- see picture validating it's coolness on left.
- cleaned my bench and austin bench
7/10 another day
- yfbE w/ and w/o rbs-ATG basic part'd (I716013, I716014)
- did an assay on wbbL/neuS - no lysing occurred with K1 phage.
- growing up for miniprep then sequencing tmrw.
7/9 MISSING OLIGOS
- So my ay013 and ay014 are missing lols, amin will order more !
- Fresh I716012 plated onto some fresh MC828E.
- i helped austin with some minipreps.
7/6 seven-six-oh-seven
- J1 and J2 are bad, J3 has a high chance of bad
- remaking I716008. digesting J3 in case it's good.
- doesn't look good. Xformed some newly made 716008.
7/5 confusing #8
- digest pcr products 1 and 2 + clean
- make kan plates
- miniprep I716011
- digest I716008 (it's bad)
- made mC828E
- send I716008 for sequencing
- make LB and agar
7/4 needs more firework
- I716011
- tryin it again...
- I716012 put in incubator
7/3 yfbe new! and other stuff
- I716012 ptet-rbs-wbbL-rbs-neuS in lo copy
- has issues with digestion. double digests look great, but triple digest still fuzzy
- two ligations of this done, one with double digest (took both fragments) and one with triple (took invisibly place where it should be)
- plated on mc868e that was saved from yesterday OMG I HOPE IT WORKS
- pcr done of yfbE, with ATG on end, and without the ATG or the rbs.
- SEE RIGHT FOR PICTURE (yfbE w/ ATG, w/o stuff, and RFP)
- speaking of RFP, the oligos I made were for a different plasmid RFP. Ohhh boy
7/2 july already?
- I716011 cm-rbs-cytB5-rbs-cytB5red plated
- I716008 ptet/rbs/wbbL/rbs/neuS (EcoRI, BamHI, AlwNI) 2146+1510+553, largest
- overnight digest test because it messed up during the day.
- will put into david plasmid tmrw.
- yfbE primers ordered. also two for getting RFP.
6/30 omg weekend rly?
- miniprep party
I716009, I716010, I716008, 9229 Right, 9203 Right, 1090 Left
6/29 public market woot
- yeaa I got up late and didn't do much today.
- cultured some cells from plates, oh boy!
- so boring I didn't even make an agenda .txt file on the comp.
6/28 /shruggery
- incubator at 25 C. wtf.
* 1-2-3ing step 1 now.. i forgot to do rbs's. lol.
- xform I716010 (kan+rbs+cytB5red)
- xform I716009 (cm+rbs+cytB5)
- 1-2-3 xform Left 1090 (rbs)
- 1-2-3 xform Right 716005 #G3 (9229)
- 1-2-3 xform Right 716006 #H? (9203)
Ayu 21:01, 28 June 2007 (EDT)
6/27 growth curve fun
- Updated registry! yayyyyy
- growth curve on yfbE/rbs/RFP
- :( no phenotype observed for iron thing.
- Sent IB and ID clones of it for sequencing.
- Xformed the aforementioned minipreps into M65 (??) cells that should turn blue (or not?)
- I716008 (Ptet-rbs-wbbL-rbs-neuS) made and xform'd
- incubated Lefty+Righty
6/26 dehumidifier machine is still loud
- Bca9229 and Bca9203 look great (G1, G2, H3, H4) (ay021, 022, 023, 024)
- poured plates
- yfbE works. like 2x. will do growth curve tmrw.
- xformed H4 into Righty, G1+G2 into Lefty
6/25 dehumidifier machine is loud
- Sent G1 and G2 for resequencing, cuz they didn't work.
- test digest yfbE try #2
- BglII/XhoI
- miniprep 9229 then test digest
- BglII/XhoI - looking for two bands
- 9229 H4,H3 sent for F sequencing w/ ca998 (023,024)
- yfbE incubated in Fe(II)SO4 instead of Fe(III)Cl3. Hope it works!
GEL: h4, h3, h2, iD, iB, iA, ladder
6/24 lol weekend
- incubated yfbE w/ and w/o Fe, and 9229.
6/22 floodrly?
- floodrly?
6/21 ok
- yfbE and neuS didn't work. wbbL was good.
- Chris redoes them all
- and all are plated. yfbE gets extra love with a 20 uL iron plate extra.
- B5 synth'd thing, miniprep'd
- Let's call it I716005
- looks ok from picture... sending G1 and G3 for forward sequencing.
- Bca9229 - B5 thing, placed into austin digest with BglII/XhoI, xformed
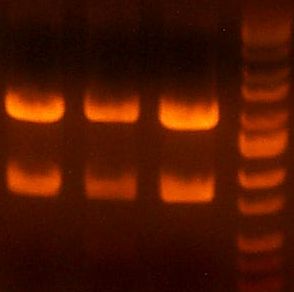
IMGS: (<< Left) The B5 reductase (?) digest looks good.
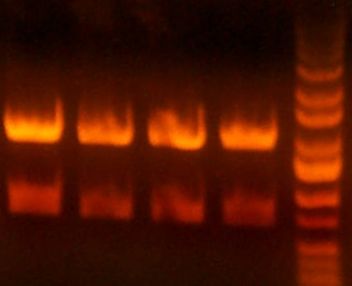
(>> Right) The digested gel to purify was good. [yfbE, neuS, 1122x3, 1121x3, wbbL]
6/20 oops
- yfbE irony thing... fail
- (BAD) w/ and w/o FeCl3 had no difference
- did mini of the F1, F2, F3 xformed and incubated stuff
- (>> digested mini with EcoRI/BamHI and got the band pattern of the parent vector (1100-1109). So failed xformation.
- I was looking for 3k and a 400, not a 3k and a 1250.
- (FIX) Got good digest of 1100-1109 from Chris, and put with new digest of yfbE to incubate on a plate.
- wbbL and neuS... no colonies on the plate (fail)
- (BAD) I believe I plated wrong.
- <<) A digestion of the miniprep looks fine (so 1121 and 1122 parent plasmids OK)
- And pretty sure that wbbL and neuS were good, and that I cut out bands right.
- (FIX) Redid incubating and plating.
Ayu 16:58, 20 June 2007 (EDT)
6/19 safety is everyone's job
- ;-)
- Sequencing received, looks good (ay05,ay06: ay016-18) see seq page
- neuS and wbbL xform'd into 1121 and 1122 libraries. Plated
- F1-4 (yfbE) incushakin, w/ and w/o FeCl3, to test promoter activity
- G1-4 incushakin: B5 (synthesized guy)
random: woot new fridge! looks quite secksy <3
6/18 speaker party
- xform'd lotta stuff
- miniprep
- pour plates
- sent wbbL for rev sequencing
- sent HPI/katG for middle sequencing ([ay06] name/ay018 prim/ay007)
other: set up speaker sys. need M-M cable. woot.
6/15 digestion party
- Good D1, D4
- synthy plasmid thingy...
# [digest] kristin B4 for backbone. Used EcoRI/XhoI purified L # [digest] synthy plasmid thingy for insert. Used EcoRI/XhoI purified S
- I716003a (pBca9145- cmr cass+rbs+neuS)
# [digest] pBca9145-neuS (I716001) (BglII, XhoI; 2063+1245; S) # [digest] pBca1101-I716051 (BamHI, XhoI; 3119+ 850; L)
- pBca9145-yfbE_pro-rbs-RFP (I716004)
# [digest] pBca9145-yfbE_promote (I716002) (EcoRI/BamHI, 2063+ 421, S) # [digest] pBca1100-Bca1109 (EcoRI/BamHI, 2927+1253, L)
- wbbL
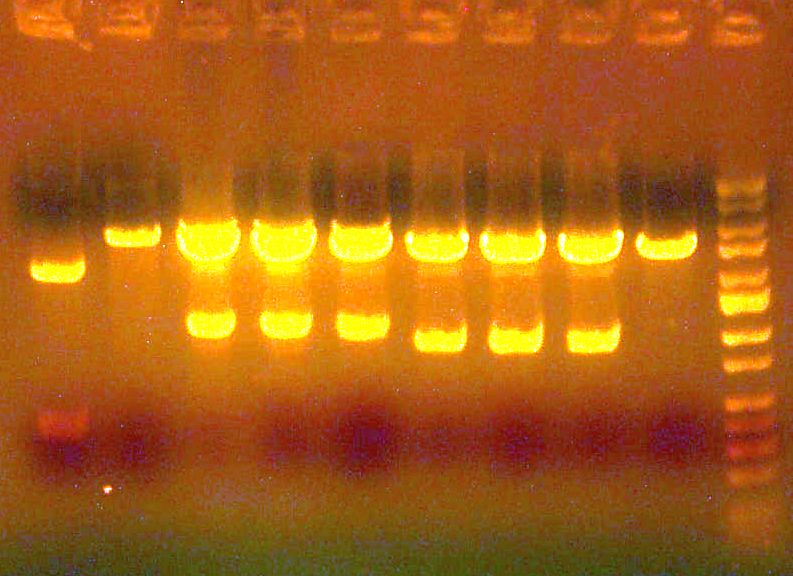
# miniprep'd and ready to go! # [IMAGE] of gel to the right: E1/E2/E3/E4/ladder >>> # Sent E1 and E2 for sequencing, forward (ay014, ay015)
NOtes: STILL NEED TO ENTER YFBE PROMOTER PART LOL entering composite parts would be nice too
I did 10 digestions today. I'm proud of myself.
6/14 stuff about things
- neuS clone C1: WE HAVE A WINNER!
- miniprep party, D1/2/3/4
- digestions didn't work too well mmmm going to sequence D1 and D4.
- wbbL good plate, now incubating in shaker
- cgctattcgcgctacctttg ready to order (middle sequencing of HPI/katG)
6/13 gloves, zymo, and ethanol oh my
- a random day
- neuS digest used to transform n plate new colonies, since the old plate had only 3 people, and 1 which worked
- wbbL digest > new plate as well (old one had one colony and it was bad)
- yfbE put into shakey tubes
- One of the neuS got miniprepped and the test digest looks good compared to test in ApE
- sending it for sequencing, eh.
- Sequencing...
- Most (A1, A4, B1) sucked
- only A3 (HPI/katG) was decent. It might have an addition mutation of a G.
- A3 sent for reverse sequencing with G01001
- Began redo-ing of HPI/katG-making, with a phase 1 PCR (the halves with a mutation)
Todo: Input parts in registry (yfbE?)
6/12 a bag full of grapes
- YAY WE ALL GOT OUR OWN SET OF PIPETTE PEOPLE
- PCR of yfbE...
- last night's thing, left in the freezer overnight. >FAIL<
- Did a new PCR -- looks good -- cells xform'd, plate is incubating.
- neuS new xformation looks good. Three colonies now incubating.
- wbbL (1) and HPI/katG (4)
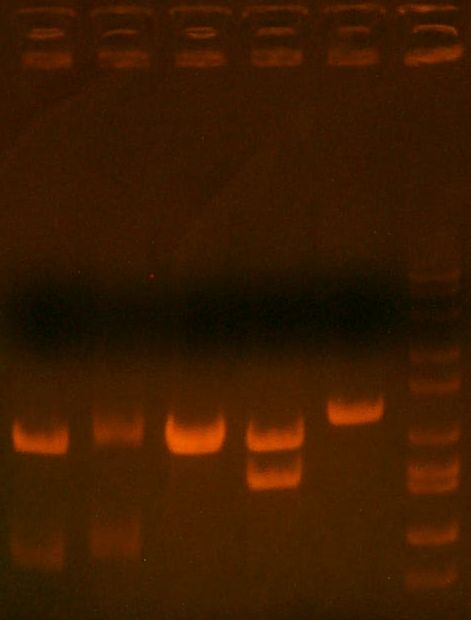
- miniprepped and digest gel ran:
- HPI/katG 1,2,3,4 || wbbL || marker
- 1,2 might be okay.. that faint band is weird. 3 is great! 4 = wtf. wbbL = wtf too (should have two bands)
- decision to put 1,3,4,wbbL for sequencing.
Ayu 17:59, 12 June 2007 (EDT)
6/11 austin's birthday
- CAKE PARTY - great custard cake
- I put the wbbL (1) and HPI/katG (4) colonies to incubate in LB broth.
- neuS failed; no colonies :(((((((
- redid ligation and xformation. hopefully there will be good results tmrw!
- made like 20 LB-Agar/Amp plates - looks like our stock will last at least this week
- researched nitric oxide (NO) and E. Coli - looks like soxRS is promising
- also researched RBCs and how they deal with NO
- plopped yfbE into PCR will do stuff with it tmrw
TO DO: enter yfbE into the registry
Ayu 18:24, 11 June 2007 (EDT)
6/8 long day?
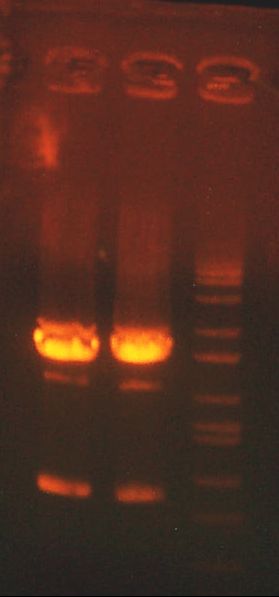
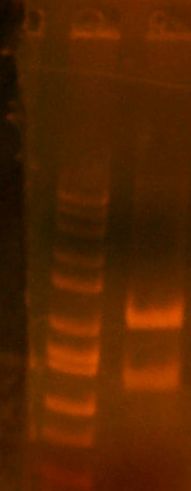
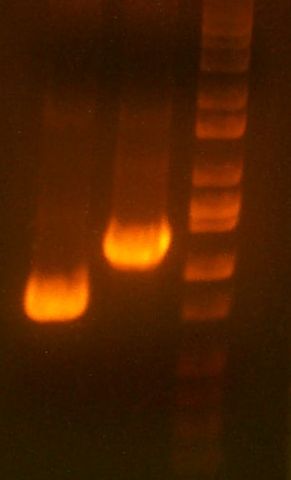
- My PCR from last night (HPI/katG) was ROXOR! (left)
- xformed some DH10B's. w00t w00t
- Today's PCR was wbbL and neuS. ALSO ROXOR LOL (right)
- xformed DH10B's.
- made oligos for yfbE promoter thingy - will test with GFP and yeah! next week!
- poured lotsa LB/agar+amp plates
6/7 we got benches
- we got benches
- pcr of [http://partsregistry.org/Part:BBa_I716253:Design HPI/katG from Salmonella]
- well... getting the mutated PCR prod overnight. going to xform tmrw, hope it works!
- programmed pcr on machine upstairs (#6)
- we got computers
- AGAR SUX, for future reference:
- nuke @ 20:00 min, 50% power.
- water bath in tap water for 5-10 min
- thaw the antibiotic right now!!
- FIRE for disinfecting
- pour that stuff. set 15 min, then marker it then bag
6/6 waiting for oligos
- Made oligos and constructs with Vai, for getting wbbL and neuS from pJ23006-Bca9106
- We tried the P_tet/RFP triple/double digest to make a composite part.
- FAIL
- probably source DNA is bad
- so much for that activity...
Other stuff: I won speed scrabble. even though I kind of cheated ish (didn't stop when Sam said stop"
6/5 coolbeans
- Finalized oligos to order with Vai
- Learned about LB broth-ing and LB/Agar plating. Thanks, Austin and Sam :)
- Learned about the many composite part-making methods. Props 2 Chris
- prefix/suffix is weaksauce
- Use the AlwnI or BsaI or BglI, in conjuction with BglII or BamHI << (Did this today)
- DBBS
- 3 antibiotic; MIT endorses, used for BioBrick 1.0. Triple digest = bad
- 1-2-3 method << 'Our Goal' in a few weeks. should be leet.
- Planned and vicariously did the making of P_tet+RFP brick (see Vai Notebook)
Other Notes: All oligos are being ordered, w00t w00t.
Ayu 18:36, 5 June 2007 (EDT)
6/4 Training Finishes, Real Stuff Starts
- Incubated some colonies
- Miniprep'd already-been-incubated colonies (2)
- Double digest of the 2 minipreps + parent plasmid
- Colony PCR'd the incubated E.coli
- Ran gel of the digest + PCR
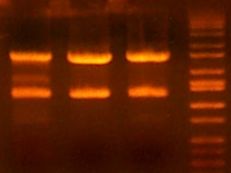
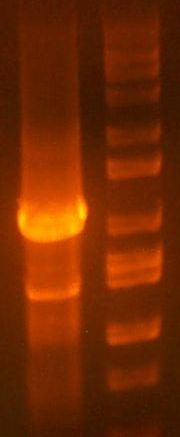
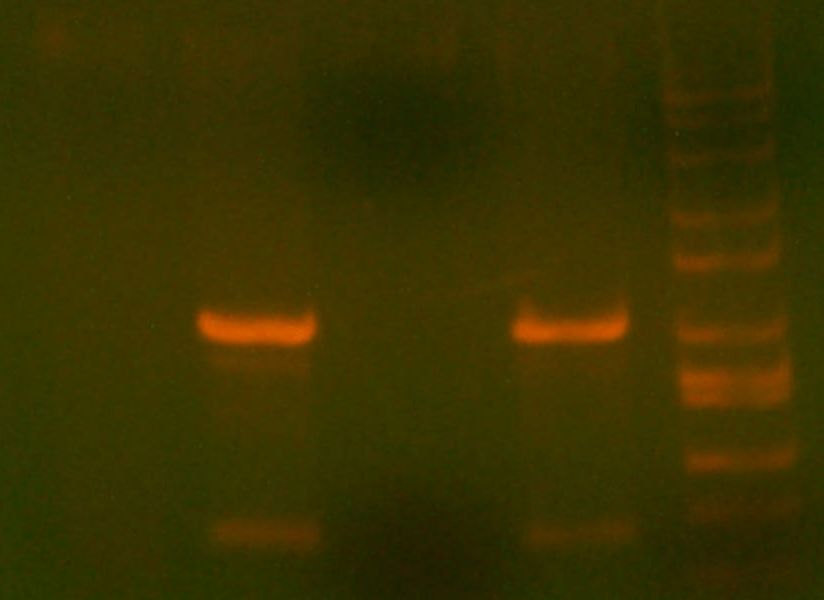
- >>> PCR product / Miniprep 1 / Parent Plasmid / Miniprep 2 / Ladder >>>
- No bands for PCR or parent. Confused? Other ones look great.
As for me: Wiki acc works now.
Designing oligos and will compare with Vai.
Ayu 18:19, 4 June 2007 (EDT)
to do