Boston University/Microencapsulation
From 2007.igem.org
| (4 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
'''Ferrozine dye:''' | '''Ferrozine dye:''' | ||
| - | Previous ferrozine dye screening methods involved the monitoring of individual S. oneidensis cells in a 384 well plate. Each well contained clear-colored ferrozine dye. The ferrozine dye turns pink when it is reduced by the bacteria. In this way, high-performing bacteria could be selected by monitoring the relative color change in the wells. Because each mutant strain must be screened individually, this process is time-consuming and inefficient. | + | Previous ferrozine dye screening methods involved the monitoring of individual ''S. oneidensis'' cells in a 384 well plate. Each well contained clear-colored ferrozine dye. The ferrozine dye turns pink when it is reduced by the bacteria. In this way, high-performing bacteria could be selected by monitoring the relative color change in the wells. Because each mutant strain must be screened individually, this process is time-consuming and inefficient. |
| + | |||
'''Microencapsulation of individual bacterium using alginate beads:''' | '''Microencapsulation of individual bacterium using alginate beads:''' | ||
| - | The newly proposed screening method involves the microencapsulation of the S. oneidensis cells with alginate beads, a seaweed extract. Using alginate beads, a single bacterium can be encapsulated in a microenvironment containing CTC, a redox-sensitive fluorescent dye. To greatly enhance the screening time of this process, fluorescence-activated cell-sorting (FACS) is then used to sort the alginate beads based on ferrozine fluorescence. | + | The newly proposed screening method involves the microencapsulation of the ''S. oneidensis'' cells with alginate beads, a seaweed extract. Using alginate beads, a single bacterium can be encapsulated in a microenvironment containing CTC, a redox-sensitive fluorescent dye. To greatly enhance the screening time of this process, fluorescence-activated cell-sorting (FACS) is then used to sort the alginate beads based on ferrozine fluorescence. |
| + | |||
| + | |||
| + | [[Image:BU_alginate.jpg|center]] | ||
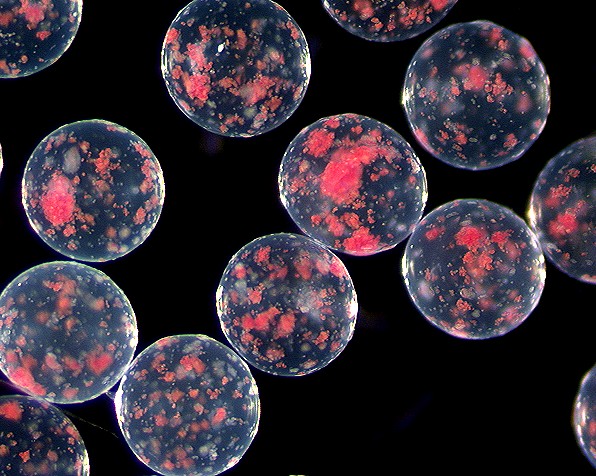
| - | + | This is an image of pancreatic islet cells encapsulated by an alginate bead. These beads are rather large and contain much than just one cell per bead. The bead diameter can vary greatly(from 25 micrometers to several millimeters). | |
[[Boston_University | Back]] | [[Boston_University | Back]] | ||
Latest revision as of 15:02, 11 July 2007
Ferrozine dye:
Previous ferrozine dye screening methods involved the monitoring of individual S. oneidensis cells in a 384 well plate. Each well contained clear-colored ferrozine dye. The ferrozine dye turns pink when it is reduced by the bacteria. In this way, high-performing bacteria could be selected by monitoring the relative color change in the wells. Because each mutant strain must be screened individually, this process is time-consuming and inefficient.
Microencapsulation of individual bacterium using alginate beads:
The newly proposed screening method involves the microencapsulation of the S. oneidensis cells with alginate beads, a seaweed extract. Using alginate beads, a single bacterium can be encapsulated in a microenvironment containing CTC, a redox-sensitive fluorescent dye. To greatly enhance the screening time of this process, fluorescence-activated cell-sorting (FACS) is then used to sort the alginate beads based on ferrozine fluorescence.
This is an image of pancreatic islet cells encapsulated by an alginate bead. These beads are rather large and contain much than just one cell per bead. The bead diameter can vary greatly(from 25 micrometers to several millimeters).