ETHZ/Biology/Lab
From 2007.igem.org
m (Put some more links.) |
(→Introduction) |
||
| (106 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | [[Image:ETHZ_banner.png|830px]] | |
| + | <!-- | ||
| + | <center>[[ETHZ | Main Page]] [[ETHZ/Model | System Modeling]] [[ETHZ/Simulation | Simulations]] [[ETHZ/Biology | System Implementation]] [[ETHZ/Biology/Lab| Lab Notes]] [[ETHZ/Meet_the_team | Meet the Team]] [[ETHZ/Internal | Team Notes]] [[ETHZ/Pictures | Pictures!]]</center><br> | ||
| + | --> | ||
| + | __NOTOC__ | ||
| + | <html> | ||
| + | <script type="text/javascript" src="http://christos.bergeles.net/eth_dropdowntabs.js"> | ||
| - | + | /*********************************************** | |
| + | * Drop Down Tabs Menu- (c) Dynamic Drive DHTML code library (www.dynamicdrive.com) | ||
| + | * This notice MUST stay intact for legal use | ||
| + | * Visit Dynamic Drive at http://www.dynamicdrive.com/ for full source code | ||
| + | ***********************************************/ | ||
| - | + | </script> | |
| - | + | <!-- CSS for Drop Down Tabs Menu #1 --> | |
| + | <link rel="stylesheet" type="text/css" href="http://christos.bergeles.net/eth_ddcolortabs.css" /> | ||
| + | <div id="colortab" class="ddcolortabs"> | ||
| + | <ul> | ||
| + | <li><a href="https://2007.igem.org/wiki/index.php?title=ETHZ" title="Home" rel="dropmenu_home"><span>Home</span></a></li> | ||
| + | <li><a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Model" title="Modeling" rel="dropmenu_modeling"><span>System Modeling</span></a></li> | ||
| + | <li><a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Simulation" title="Simulations" rel="dropmenu_simulation"><span>Simulations</span></a></li> | ||
| + | <li><a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Biology" title="System Implementation" rel="dropmenu_biology"><span>System Implementation</span></a></li> | ||
| + | <li><a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Meet_the_team" title="Meet the team" rel="dropmenu_meettheteam"><span>Meet the team</span></a></li> | ||
| + | <li><a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Pictures" title="Pictures!" rel="dropmenu_pictures"><span>Pictures!</span></a></li> | ||
| + | </ul> | ||
| + | </div> | ||
| + | <div class="ddcolortabsline"> </div> | ||
| - | |||
| - | + | <!--1st drop down menu --> | |
| + | <div id="dropmenu_home" class="dropmenudiv_a"> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ#Introduction">Introduction</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ#Team_Members">Team Members</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ#Acknowledgments">Acknowledgments</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ#Site_Map">Site map</a> | ||
| + | </div> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | == | + | <!--2nd drop down menu --> |
| + | <div id="dropmenu_modeling" class="dropmenudiv_a" style="width: 150px;"> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Model#Introduction">Introduction</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Model#Model_Overview">Model Overview</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Model#Detailed_Model">Detailed Model</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Model#Final_Model">Final Model</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Modeling_Basics">Modeling Basics Page</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Model#Mathematical_Model">Mathematical Model</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/FSM">FSM View Page</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/FlipFlop">Flip-Flop View Page</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Parameters">Parameters Page</a> | ||
| + | </div> | ||
| - | === | + | <!--3rd drop down menu --> |
| + | <div id="dropmenu_simulation" class="dropmenudiv_a" style="width: 150px;"> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Simulation#Introduction">Introduction</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Simulation#Simulation_of_Test_Cases">Test Cases</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Simulation#Sensitivity_Analysis">Sensitivity Analysis</a> | ||
| + | </div> | ||
| - | + | <!--4th drop down menu --> | |
| + | <div id="dropmenu_biology" class="dropmenudiv_a" style="width: 150px;"> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Biology#Introduction">Introduction</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Biology#The_Complete_System">The Complete System</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Biology#System_Phases">System Phases</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Biology#Current_Cloning_Status">Current Cloning Status</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Biology/parts">System Parts Page</a> | ||
| + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Biology/Lab">Lab Notes Page</a> | ||
| + | </div> | ||
| - | + | <!--5th drop down menu --> | |
| - | + | <div id="dropmenu_meettheteam" class="dropmenudiv_a" style="width: 150px;"> | |
| - | + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Meet_the_team#The_ETH_Zurich_07_Team">The ETH Zurich 07 Team</a> | |
| - | + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Meet_the_team#Team_Description">Team Description</a> | |
| - | + | <a href="https://2007.igem.org/wiki/index.php?title=ETHZ/Internal">Brainstorming Page</a> | |
| + | </div> | ||
| - | + | <script type="text/javascript"> | |
| - | + | //SYNTAX: tabdropdown.init("menu_id", [integer OR "auto"]) | |
| + | tabdropdown.init("colortab", 3) | ||
| + | </script> | ||
| - | + | </html> | |
| - | + | __NOTOC__ | |
| - | + | = Introduction = | |
| - | + | ||
| - | + | ||
| - | + | On this page, you can find information about how educatETH <i>E.coli</i> was implemented in the lab. More specifically, you will find information on the plasmid strains we used, the modifications we did to them in order to be compatible with the Biobrick library and our cloning plan. Moreover, you can find [[ETHZ/Biology/Labbook| here]] an (unfortunately not complete) electronic copy of our lab notebook. If you are here because you are interested in implementing educatETH <i>E.coli</i> in your lab, then our System Implementation and the System Parts pages may be of help to you! | |
| - | + | For all our cloning procedures we used standard protocols according to ''SAMBROOK and RUSSELL'' Molecular Cloning: A Laboratory Manual [1]. | |
| - | + | == Strains == | |
| - | + | We used the following <i>E. coli </i> strains: | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | [http://openwetware.org/wiki/E._coli_genotypes#TOP10_.28Invitrogen.29|<b><i>E. coli </i>Top10 (Invitrogen):</b>] <br> | |
| - | + | *You can find this strain at [http://partsregistry.org/Part:BBa_V1009 BBa_V1009] | |
| - | + | *This strain has a streptomycin resistance <br> | |
| - | + | *Genotype: F’ {tetR}, mcrA, Δ(mrr-hsdRMS-mcrBC), φ80 lacZ ΔM15, ΔlacX74, deoR, recA1, araD139 Δ(ara-leu)7679, galU, galK, λ-, rpsL,endA1, nupG | |
| + | *For further information please [http://openwetware.org/wiki/E._coli_genotypes#TOP10_.28Invitrogen.29| click here] | ||
| + | *<i>References</i>: | ||
| + | **Casdaban, M. and Cohen, S. (1980) J Mol Biol 138:179 PMID 6997493 <br> | ||
| + | **Grant, S.G.N. et al. (1990) Proc. Natl. Acad. Sci. USA 87: 4645-4649 PMID 2162051 | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | [http://openwetware.org/wiki/E._coli_genotypes#JM101|<b><i>E. coli </i>JM101:</b>] <br> | |
| + | *You can find this strain at [http://partsregistry.org/Part:BBa_I739301 BBa_I739301] | ||
| + | *We call them <i>Jimmys</i> | ||
| + | *This strain is the original blue/white cloning strain | ||
| + | *Genotype: glnV44, thi-1, Δ(lac-proAB), F'[lacIqZΔM15 traD36 proAB+] | ||
| + | *For further information please [http://openwetware.org/wiki/E._coli_genotypes#JM101| click here] | ||
| + | *<i>Reference</i>: | ||
| + | **Messing, J. et al. (1981) Nucleic Acids Res. 9, 309; Yanisch-Perron, C., Vieira, J., and Messing, J. (1985) Gene 33, 103 | ||
| - | |||
| - | + | == Plasmids == | |
| + | For our system we needed three plasmids with different origins of replication and antibiotic resistances. We decided to take low copy plasmids. We also decided to use the following plasmids, which we wanted to modify so that they would become compatible to the Biobrick Library multiple cloning site: | ||
| - | |||
| - | + | === Basic plasmids === | |
| - | + | ||
| - | + | ||
| - | ===== | + | {| class="wikitable" border="1" cellspacing="0" cellpadding="2" style="text-align:left; margin: 1em 1em 1em 0; background: #f9f9f9; border: 1px #aaa solid; border-collapse: collapse;" |
| + | |- | ||
| + | ! Plasmid !! Resistances !! Copy number !! Origin !! Map | ||
| + | |- | ||
| + | | [[ETHZ/pbr322| pBR322]] [2,3] || width=505px | Ampicillin, Tetracyline || 15-20 [4] || width=96px | pMB1 || [[Image:Mappbr322.jpg|center|thumb|pBR322 Map|100px]] | ||
| + | |- | ||
| + | | [[ETHZ/pck01| pCK01]] [5] || Chloramphenicol|| 5-12 [4] || pSC101 || [[Image:Mappck01.jpg|center|thumb|pCK01 Map|100px]] | ||
| + | |- | ||
| + | | [[ETHZ/pacyc177| pACYC177]] [6,7] || Ampicillin, Kanamycin|| 10-12 [4] || p15A || [[Image:Mappacyc177.jpg|center|thumb|pACYC177 Map|100px]] | ||
| + | |- | ||
| + | |} | ||
| + | <br> | ||
| - | + | === Changes to the plasmids === | |
| - | + | In order to get the Biobrick multiple cloning site into the plasmids, we had to make several changes to the plasmids: | |
| - | + | ||
| - | + | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{| class="wikitable" border="1" cellspacing="0" cellpadding="2" style="text-align:left; margin: 1em 1em 1em 0; background: #f9f9f9; border: 1px #aaa solid; border-collapse: collapse;" | {| class="wikitable" border="1" cellspacing="0" cellpadding="2" style="text-align:left; margin: 1em 1em 1em 0; background: #f9f9f9; border: 1px #aaa solid; border-collapse: collapse;" | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|- | |- | ||
| - | | | + | ! Plasmid !! Changes !! width=85px| New name !! New resistance !! New Map |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|- | |- | ||
| - | | | + | | width=94px | [http://partsregistry.org/Part:BBa_I739201 BBa_I739201] |
| - | | | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| | | | ||
| - | * | + | *Site directed mutagenesis: Changed the GCA codon of the PstI site of the bla gene into GTA |
| - | + | *Cloned in [[ETHZ/pbr322-3| linker oligos]] (EcoRI/BamHI) | |
| - | + | | | |
| - | | | + | [[ETHZ/pbr322| pBR322]] |
| - | + | ||
| - | + | ||
| - | + | ||
| - | | | + | |
| - | + | ||
| - | + | ||
| | | | ||
| - | + | Ampicillin | |
| + | | | ||
| + | [[Image:MapI739201.jpg|center|thumb|BBa_I739201 Map|100px]] | ||
|- | |- | ||
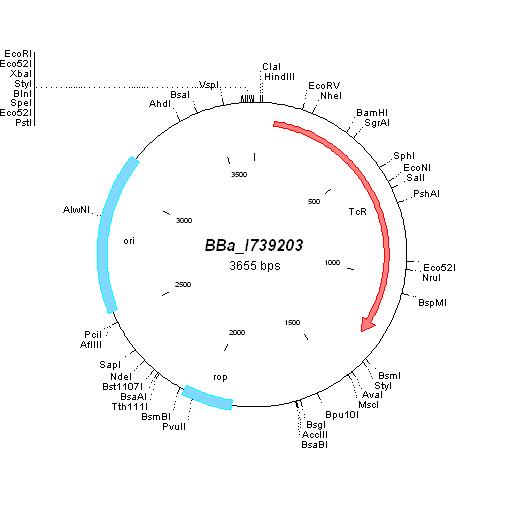
| - | | | + | | [http://partsregistry.org/Part:BBa_I739203 BBa_I739203] |
| | | | ||
| - | * | + | *Cloned in [[ETHZ/pbr322-1| linker oligos]] (EcoRI/PstI) |
| - | + | | | |
| - | | | + | [[ETHZ/pbr322| pBR322]] |
| - | + | | | |
| - | + | Tetracycline | |
| - | + | | | |
| - | + | [[Image:MapI739203.jpg|center|thumb|BBa_I739203 Map|100px]] | |
| - | | | + | |
|- | |- | ||
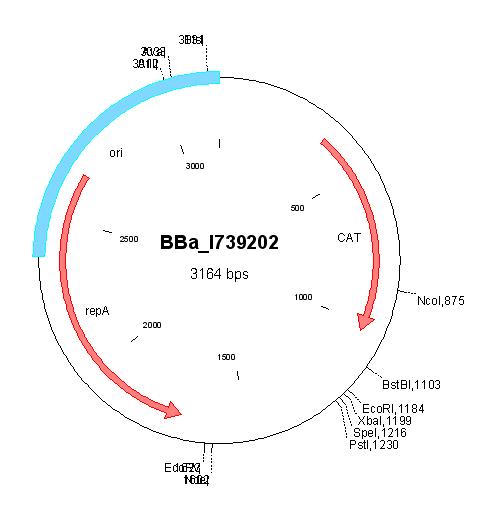
| - | | | + | | [http://partsregistry.org/Part:BBa_I739202 BBa_I739202] |
| - | | | + | | |
| - | | | + | *[[ETHZ/primer_pcr_killspe_up| Site directed mutagenesis]]: Changed the ACT codon of the SpeI site in the origin of replication into ATT |
| + | *Cloned in [[ETHZ/pck01-3| linker oligos]] (AgeI/AseI) | ||
| + | | | ||
| + | [[ETHZ/pck01| pCK01]] | ||
| + | | | ||
| + | Chloramphenicol | ||
| | | | ||
| + | [[Image:MapI739202.jpg|center|thumb|BBa_I739202 Map|100px]] | ||
|- | |- | ||
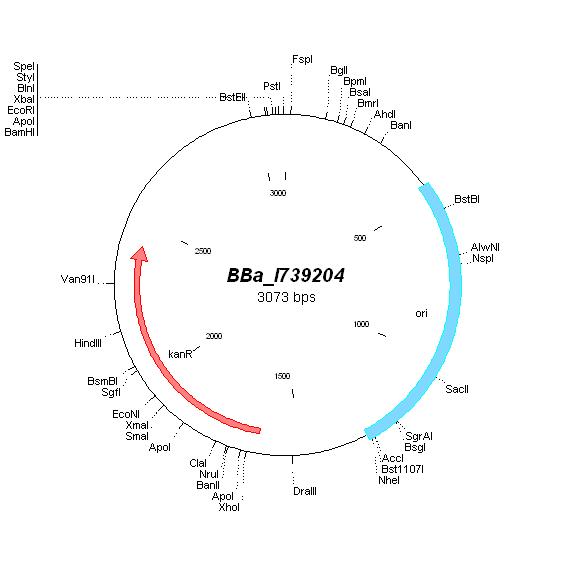
| - | | | + | | [http://partsregistry.org/Part:BBa_I739204 BBa_I739204] |
| | | | ||
| + | *Cloned in [[ETHZ/pacyc177-1| linker oligos]] (BamHI/PstI) | ||
| | | | ||
| + | [[ETHZ/pacyc177| pACYC177]] | ||
| | | | ||
| + | Kanamycin | ||
| + | | [[Image:MapI739204.jpg|center|thumb|BBa_I739204 Map|100px]] | ||
| + | |- | ||
|} | |} | ||
| + | <br> | ||
| - | ==== | + | ==Cloning plan== |
| - | + | ||
| - | + | ||
| - | === | + | ===Parts assignment into plasmids=== |
| + | The plan was to put the following parts into the three plasmids (for the detailed cloning procedure see below): | ||
{| class="wikitable" border="1" cellspacing="0" cellpadding="2" style="text-align:left; margin: 1em 1em 1em 0; background: #f9f9f9; border: 1px #aaa solid; border-collapse: collapse;" | {| class="wikitable" border="1" cellspacing="0" cellpadding="2" style="text-align:left; margin: 1em 1em 1em 0; background: #f9f9f9; border: 1px #aaa solid; border-collapse: collapse;" | ||
| - | + | |- | |
| - | + | ! plasmid !! resistance !! copy type!! contents !! comments | |
| - | + | |- | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | | [http://partsregistry.org/Part:BBa_I739201 BBa_I739201] || ampicillin || low || [http://partsregistry.org/Part:BBa_I739001 BBa_I739001(TetR) ], [http://partsregistry.org/Part:BBa_I739002 BBa_I739002(LacI) ], [http://partsregistry.org/Part:BBa_I739003 BBa_I739003(LuxR) ] || constitutive subsystem | |
| + | |- | ||
| - | + | | [http://partsregistry.org/Part:BBa_I739202 BBa_I739202] || chloramphenicol|| low || [http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII) ], [http://partsregistry.org/Part:BBa_E0430 BBa_E0430(EYFP) ], [http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI) ], [http://partsregistry.org/Part:BBa_I739009 BBa_I739009(ECFP) ] || reporting subsystem | |
| - | + | |- | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | | [http://partsregistry.org/Part:BBa_I739204 BBa_I739204] || kanamycin|| low || [http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI) ], [http://partsregistry.org/Part:BBa_I739007 BBa_I739007(P22 cII) ], [http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP) ], [http://partsregistry.org/Part:BBa_I739011 BBa_I739011(GFP) ] || learning subsystem, reporting subsystem | |
| + | |- | ||
| + | |} | ||
| - | + | It is important to insert parts responsible for the production of fluorescent proteins in low copy plasmids, as they are potentially harmful for the cell. Unfortunately, working with low copy plasmids makes the procedure more demanding in the lab. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | |||
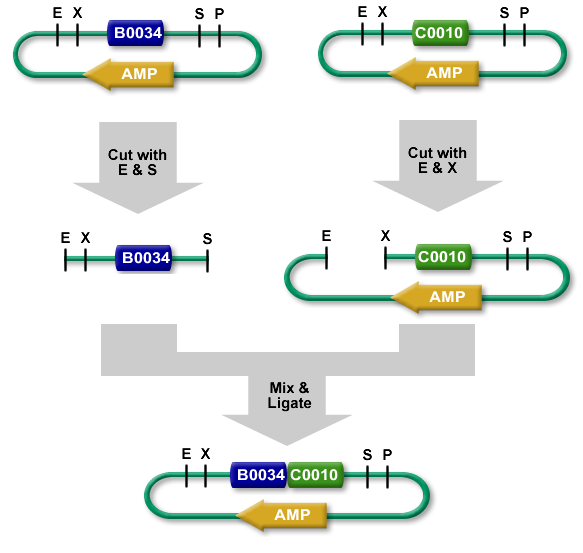
| - | + | === Cloning procedure === | |
| - | + | The standard BioBrick assembly will be used to put the parts in the plasmids. Detailed information on how the BioBrick part fabrication works can be found [http://openwetware.org/wiki/Synthetic_Biology:BioBricks/Part_fabrication here]. For a shorter explanation of how to assemble 2 parts together check [http://partsregistry.org/Assembly:Standard_assembly here]. [[Image:Assembly _process.png|thumb|300px|DNA assembly process [8]]] Note that the composite part is constructed from the end to the beginning, i.e. each new part is inserted ''before'' the existing one. Composite parts made of parts '''a''' and '''b''' are denoted '''a.b'''. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | ===='' | + | ==== Plasmid 1 ''([http://partsregistry.org/Part:BBa_I739201 BBa_I739201])'' ==== |
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | # Digest '''[http://partsregistry.org/Part:BBa_I739001 I739001(TetR)]''' and [http://partsregistry.org/Part:BBa_I739201 BBa_I739201] plasmid with EcoRI/PstI and ligate them afterwards. | |
| + | # Digest '''[http://partsregistry.org/Part:BBa_I739002 I739002(LacI)]''' and '''[http://partsregistry.org/Part:BBa_I739003 I739003(LuxR)]''' with XbaI/PstI | ||
| + | # Digest '''[http://partsregistry.org/Part:BBa_I739001 I739001(TetR)]''' in [http://partsregistry.org/Part:BBa_I739201 BBa_I739201] with SpeI/PstI. | ||
| + | # Ligate digested '''[http://partsregistry.org/Part:BBa_I739001 I739001(TetR)]''' in [http://partsregistry.org/Part:BBa_I739201 BBa_I739201] with digested '''[http://partsregistry.org/Part:BBa_I739002 I739002(LacI)]'''. You get a plasmid containing a '''[http://partsregistry.org/Part:BBa_I739001 I739001(TetR)].[http://partsregistry.org/Part:BBa_I739002 I739002(LacI)]''' composite part. | ||
| + | # Digest '''[http://partsregistry.org/Part:BBa_I739001 I739001(TetR)].[http://partsregistry.org/Part:BBa_I739002 I739002(LacI)]''' with SpeI/PstI. | ||
| + | # Ligate digested '''[http://partsregistry.org/Part:BBa_I739001 I739001(TetR)].[http://partsregistry.org/Part:BBa_I739002 I739002(LacI)]''' with digested '''[http://partsregistry.org/Part:BBa_I739003 I739003(LuxR)]'''. You get the completed '''[http://partsregistry.org/Part:BBa_I739001 I739001(TetR)].[http://partsregistry.org/Part:BBa_I739002 I739002(LacI)].[http://partsregistry.org/Part:BBa_I739003 I739003(LuxR)]''' composite part in the [http://partsregistry.org/Part:BBa_I739201 BBa_I739201] plasmid. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | + | ==== Plasmid 2 ''([http://partsregistry.org/Part:BBa_I739202 BBa_I739202])''==== | |
| - | + | ||
| - | + | ||
| - | + | # Digest '''[http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII)]''', '''[http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI)]''' and the plasmid [http://partsregistry.org/Part:BBa_I739202 BBa_I739202] with EcoRI/PstI. | |
| - | + | # Digest '''[http://partsregistry.org/Part:BBa_E0430 BBa_E0430(EYFP)]''' and '''[http://partsregistry.org/Part:BBa_I739009 BBa_I739009(ECFP)]''' with XbaI/PstI. | |
| - | + | # Ligate digested '''[http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII)]''' and digested plasmid [http://partsregistry.org/Part:BBa_I739202 BBa_I739202]. | |
| - | + | # Ligate digested '''[http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI)]''' and the plasmid [http://partsregistry.org/Part:BBa_I739202 BBa_I739202]. | |
| - | + | # Digest '''[http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII)]''' in [http://partsregistry.org/Part:BBa_I739202 BBa_I739202] with SpeI/PstI. | |
| - | + | # Digest '''[http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI)]''' in [http://partsregistry.org/Part:BBa_I739202 BBa_I739202] with SpeI/PstI. | |
| - | + | # Ligate digested '''[http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII)]''' in [http://partsregistry.org/Part:BBa_I739202 BBa_I739202] with digested '''[http://partsregistry.org/Part:BBa_E0430 BBa_E0430(EYFP)]'''. You get a plasmid containing a '''[http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII)].[http://partsregistry.org/Part:BBa_E0430 BBa_E0430(EYFP)]''' composite part. | |
| - | + | # Ligate digested '''[http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI)]''' in [http://partsregistry.org/Part:BBa_I739202 BBa_I739202] with '''[http://partsregistry.org/Part:BBa_I739009 BBa_I739009(ECFP)]'''. You get a plasmid containing a '''[http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI)].[http://partsregistry.org/Part:BBa_I739009 BBa_I739009(ECFP)]''' composite part. | |
| - | + | # Digest '''[http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII)].[http://partsregistry.org/Part:BBa_E0430 BBa_E0430(EYFP)]''' with SpeI/PstI. | |
| - | + | # Digest '''[http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI)].[http://partsregistry.org/Part:BBa_I739009 BBa_I739009(ECFP)]''' with XbaI/PstI. | |
| - | + | # Ligate digested '''[http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII)].[http://partsregistry.org/Part:BBa_E0430 BBa_E0430(EYFP)]''' and digested '''[http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI)].[http://partsregistry.org/Part:BBa_I739009 BBa_I739009(ECFP)]'''. You get the completed plasmid containing the '''[http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII)].[http://partsregistry.org/Part:BBa_E0430 BBa_E0430(EYFP)].[http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI)].[http://partsregistry.org/Part:BBa_I739009 BBa_I739009(ECFP)]''' composite part. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ====Plasmid 3 ''([http://partsregistry.org/Part:BBa_I739204 BBa_I739204])''==== | |
| - | + | ||
| - | + | ||
| - | + | # Digest '''[http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI)]''', '''[http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP)]''' and the plasmid [http://partsregistry.org/Part:BBa_I739204 BBa_I739204] with EcoRI/PstI. | |
| - | + | # Digest '''[http://partsregistry.org/Part:BBa_I739007 BBa_I739007(P22 cII)]''' and '''[http://partsregistry.org/Part:BBa_I739011 BBa_I739011(GFP)]''' with XbaI/PstI. | |
| - | + | # Ligate digested '''[http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI)]''' and digested plasmid [http://partsregistry.org/Part:BBa_I739204 BBa_I739204]. | |
| - | + | # Ligate digested '''[http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP)]''' and the plasmid [http://partsregistry.org/Part:BBa_I739204 BBa_I739204]. | |
| - | + | # Digest '''[http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI)]''' in [http://partsregistry.org/Part:BBa_I739204 BBa_I739204] with SpeI/PstI. | |
| - | + | # Digest '''[http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP)]''' in [http://partsregistry.org/Part:BBa_I739204 BBa_I739204] with SpeI/PstI. | |
| - | + | # Ligate digested '''[http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI)]''' in [http://partsregistry.org/Part:BBa_I739204 BBa_I739204] with digested '''[http://partsregistry.org/Part:BBa_I739007 BBa_I739007(P22 cII)]'''. You get a plasmid containing a '''[http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI)].[http://partsregistry.org/Part:BBa_I739007 BBa_I739007(P22 cII)]''' composite part. | |
| - | + | # Ligate digested '''[http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP)]''' in [http://partsregistry.org/Part:BBa_I739204 BBa_I739204] with '''[http://partsregistry.org/Part:BBa_I739011 BBa_I739011(GFP)]'''. You get a plasmid containing a '''[http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP)].[http://partsregistry.org/Part:BBa_I739011 BBa_I739011(GFP)]''' composite part. | |
| - | + | # Digest '''[http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI)].[http://partsregistry.org/Part:BBa_I739007 BBa_I739007(P22 cII)]''' with SpeI/PstI. | |
| - | + | # Digest '''[http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP)].[http://partsregistry.org/Part:BBa_I739011 BBa_I739011(GFP)]''' with XbaI/PstI. | |
| - | + | # Ligate digested '''[http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI)].[http://partsregistry.org/Part:BBa_I739007 BBa_I739007(P22 cII)]''' and digested '''[http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP)].[http://partsregistry.org/Part:BBa_I739011 BBa_I739011(GFP)]'''. You get the completed plasmid containing the '''[http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI)].[http://partsregistry.org/Part:BBa_I739007 BBa_I739007(P22 cII)].[http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP)].[http://partsregistry.org/Part:BBa_I739011 BBa_I739011(GFP)]''' composite part. | |
| - | + | <br> | |
| - | + | ||
| - | + | ==References== | |
| - | + | ||
| - | + | [http://www.MolecularCloning.com [1] Sambrook J and Russel DW] <i>"Molecular Cloning: A Laboratory Manual"</i>, Cold Spring Harbour Laboratory Press, 3rd edition, 2001<br /> | |
| - | + | [2] Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heynecker HL and Boyer HW <i>"Construction of useful cloning vectors"</i>, Gene 2: 95-113, 1977<br /> | |
| - | + | [3] Watson N <i>"A new revision of the sequence of plasmid pBR322"</i>, Gene 70: 399-403, 1988<br /> | |
| - | + | [http://www1.qiagen.com/faq/faqview.aspx?faqid=350&SearchText=&FaqCategoryId=0&MenuItemId=0&catalog=1&ProductLineId=1000228 [4] QIAGEN FAQs]<br /> | |
| - | + | [http://www.blackwell-synergy.com/doi/abs/10.1111/j.1365-2958.1995.tb02293.x [5] Fernández S et al.] <i>"Activation of the transcriptional regulator XylR of Pseudomonas putida by release of repression between functional domains"</i>, Mol Microbiol 16(2):205-213, 1995]<br /> | |
| - | + | [6] Chang ACY and Cohen SN <i>"Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid"</i>, J Bacteriol 134: 1141-1156, 1978<br /> | |
| + | [7] Rose, R.E., <i>"The nucleotide sequence of pACYC177"</i>, Nucleic Acids Res, 16(1): 356, 1988<br /> | ||
| + | [http://partsregistry.org/Assembly:Standard_assembly [8] Standard Assembly Process]<br /> | ||
Latest revision as of 20:03, 26 October 2007
Introduction
On this page, you can find information about how educatETH E.coli was implemented in the lab. More specifically, you will find information on the plasmid strains we used, the modifications we did to them in order to be compatible with the Biobrick library and our cloning plan. Moreover, you can find here an (unfortunately not complete) electronic copy of our lab notebook. If you are here because you are interested in implementing educatETH E.coli in your lab, then our System Implementation and the System Parts pages may be of help to you!
For all our cloning procedures we used standard protocols according to SAMBROOK and RUSSELL Molecular Cloning: A Laboratory Manual [1].
Strains
We used the following E. coli strains:
[http://openwetware.org/wiki/E._coli_genotypes#TOP10_.28Invitrogen.29|E. coli Top10 (Invitrogen):]
- You can find this strain at [http://partsregistry.org/Part:BBa_V1009 BBa_V1009]
- This strain has a streptomycin resistance
- Genotype: F’ {tetR}, mcrA, Δ(mrr-hsdRMS-mcrBC), φ80 lacZ ΔM15, ΔlacX74, deoR, recA1, araD139 Δ(ara-leu)7679, galU, galK, λ-, rpsL,endA1, nupG
- For further information please [http://openwetware.org/wiki/E._coli_genotypes#TOP10_.28Invitrogen.29| click here]
- References:
- Casdaban, M. and Cohen, S. (1980) J Mol Biol 138:179 PMID 6997493
- Grant, S.G.N. et al. (1990) Proc. Natl. Acad. Sci. USA 87: 4645-4649 PMID 2162051
- Casdaban, M. and Cohen, S. (1980) J Mol Biol 138:179 PMID 6997493
[http://openwetware.org/wiki/E._coli_genotypes#JM101|E. coli JM101:]
- You can find this strain at [http://partsregistry.org/Part:BBa_I739301 BBa_I739301]
- We call them Jimmys
- This strain is the original blue/white cloning strain
- Genotype: glnV44, thi-1, Δ(lac-proAB), F'[lacIqZΔM15 traD36 proAB+]
- For further information please [http://openwetware.org/wiki/E._coli_genotypes#JM101| click here]
- Reference:
- Messing, J. et al. (1981) Nucleic Acids Res. 9, 309; Yanisch-Perron, C., Vieira, J., and Messing, J. (1985) Gene 33, 103
Plasmids
For our system we needed three plasmids with different origins of replication and antibiotic resistances. We decided to take low copy plasmids. We also decided to use the following plasmids, which we wanted to modify so that they would become compatible to the Biobrick Library multiple cloning site:
Basic plasmids
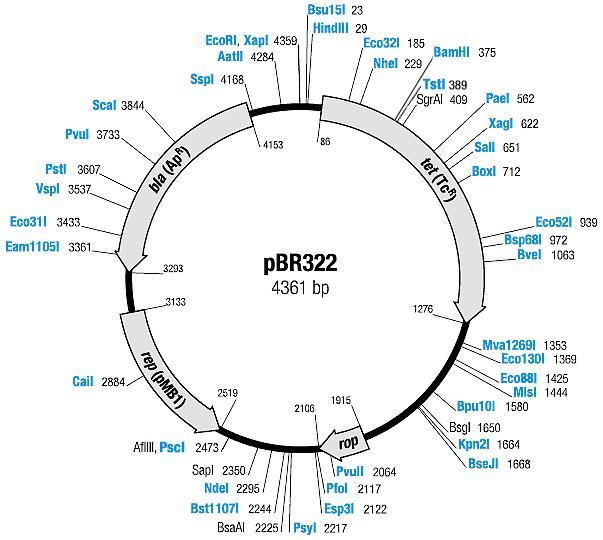
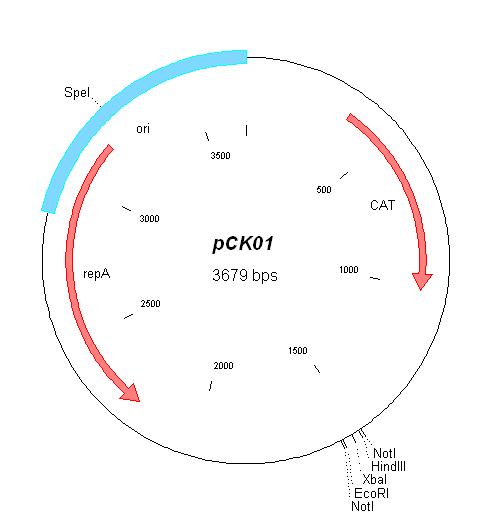
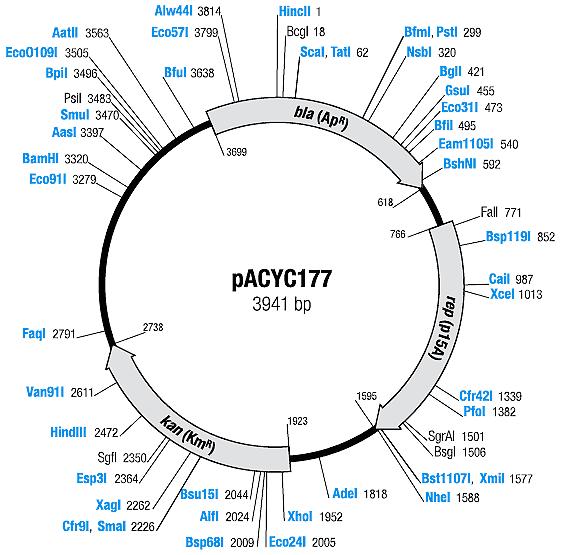
| Plasmid | Resistances | Copy number | Origin | Map |
|---|---|---|---|---|
| pBR322 [2,3] | Ampicillin, Tetracyline | 15-20 [4] | pMB1 | |
| pCK01 [5] | Chloramphenicol | 5-12 [4] | pSC101 | |
| pACYC177 [6,7] | Ampicillin, Kanamycin | 10-12 [4] | p15A |
Changes to the plasmids
In order to get the Biobrick multiple cloning site into the plasmids, we had to make several changes to the plasmids:
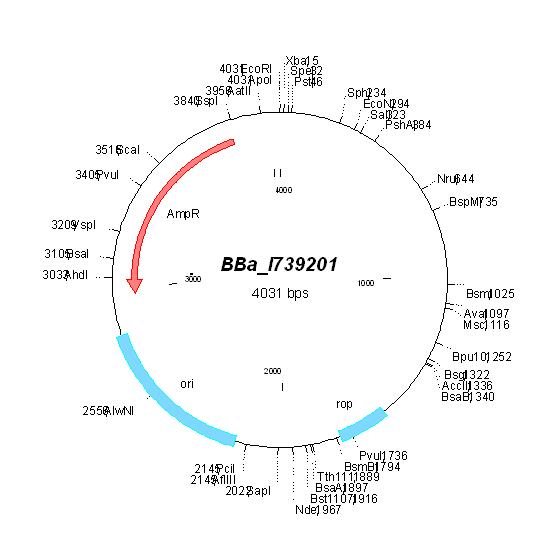
| Plasmid | Changes | New name | New resistance | New Map |
|---|---|---|---|---|
| [http://partsregistry.org/Part:BBa_I739201 BBa_I739201] |
|
Ampicillin | ||
| [http://partsregistry.org/Part:BBa_I739203 BBa_I739203] |
|
Tetracycline | ||
| [http://partsregistry.org/Part:BBa_I739202 BBa_I739202] |
|
Chloramphenicol | ||
| [http://partsregistry.org/Part:BBa_I739204 BBa_I739204] |
|
Kanamycin |
Cloning plan
Parts assignment into plasmids
The plan was to put the following parts into the three plasmids (for the detailed cloning procedure see below):
| plasmid | resistance | copy type | contents | comments |
|---|---|---|---|---|
| [http://partsregistry.org/Part:BBa_I739201 BBa_I739201] | ampicillin | low | [http://partsregistry.org/Part:BBa_I739001 BBa_I739001(TetR) ], [http://partsregistry.org/Part:BBa_I739002 BBa_I739002(LacI) ], [http://partsregistry.org/Part:BBa_I739003 BBa_I739003(LuxR) ] | constitutive subsystem |
| [http://partsregistry.org/Part:BBa_I739202 BBa_I739202] | chloramphenicol | low | [http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII) ], [http://partsregistry.org/Part:BBa_E0430 BBa_E0430(EYFP) ], [http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI) ], [http://partsregistry.org/Part:BBa_I739009 BBa_I739009(ECFP) ] | reporting subsystem |
| [http://partsregistry.org/Part:BBa_I739204 BBa_I739204] | kanamycin | low | [http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI) ], [http://partsregistry.org/Part:BBa_I739007 BBa_I739007(P22 cII) ], [http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP) ], [http://partsregistry.org/Part:BBa_I739011 BBa_I739011(GFP) ] | learning subsystem, reporting subsystem |
It is important to insert parts responsible for the production of fluorescent proteins in low copy plasmids, as they are potentially harmful for the cell. Unfortunately, working with low copy plasmids makes the procedure more demanding in the lab.
Cloning procedure
The standard BioBrick assembly will be used to put the parts in the plasmids. Detailed information on how the BioBrick part fabrication works can be found [http://openwetware.org/wiki/Synthetic_Biology:BioBricks/Part_fabrication here]. For a shorter explanation of how to assemble 2 parts together check [http://partsregistry.org/Assembly:Standard_assembly here]. Note that the composite part is constructed from the end to the beginning, i.e. each new part is inserted before the existing one. Composite parts made of parts a and b are denoted a.b.
Plasmid 1 ([http://partsregistry.org/Part:BBa_I739201 BBa_I739201])
- Digest [http://partsregistry.org/Part:BBa_I739001 I739001(TetR)] and [http://partsregistry.org/Part:BBa_I739201 BBa_I739201] plasmid with EcoRI/PstI and ligate them afterwards.
- Digest [http://partsregistry.org/Part:BBa_I739002 I739002(LacI)] and [http://partsregistry.org/Part:BBa_I739003 I739003(LuxR)] with XbaI/PstI
- Digest [http://partsregistry.org/Part:BBa_I739001 I739001(TetR)] in [http://partsregistry.org/Part:BBa_I739201 BBa_I739201] with SpeI/PstI.
- Ligate digested [http://partsregistry.org/Part:BBa_I739001 I739001(TetR)] in [http://partsregistry.org/Part:BBa_I739201 BBa_I739201] with digested [http://partsregistry.org/Part:BBa_I739002 I739002(LacI)]. You get a plasmid containing a [http://partsregistry.org/Part:BBa_I739001 I739001(TetR)].[http://partsregistry.org/Part:BBa_I739002 I739002(LacI)] composite part.
- Digest [http://partsregistry.org/Part:BBa_I739001 I739001(TetR)].[http://partsregistry.org/Part:BBa_I739002 I739002(LacI)] with SpeI/PstI.
- Ligate digested [http://partsregistry.org/Part:BBa_I739001 I739001(TetR)].[http://partsregistry.org/Part:BBa_I739002 I739002(LacI)] with digested [http://partsregistry.org/Part:BBa_I739003 I739003(LuxR)]. You get the completed [http://partsregistry.org/Part:BBa_I739001 I739001(TetR)].[http://partsregistry.org/Part:BBa_I739002 I739002(LacI)].[http://partsregistry.org/Part:BBa_I739003 I739003(LuxR)] composite part in the [http://partsregistry.org/Part:BBa_I739201 BBa_I739201] plasmid.
Plasmid 2 ([http://partsregistry.org/Part:BBa_I739202 BBa_I739202])
- Digest [http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII)], [http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI)] and the plasmid [http://partsregistry.org/Part:BBa_I739202 BBa_I739202] with EcoRI/PstI.
- Digest [http://partsregistry.org/Part:BBa_E0430 BBa_E0430(EYFP)] and [http://partsregistry.org/Part:BBa_I739009 BBa_I739009(ECFP)] with XbaI/PstI.
- Ligate digested [http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII)] and digested plasmid [http://partsregistry.org/Part:BBa_I739202 BBa_I739202].
- Ligate digested [http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI)] and the plasmid [http://partsregistry.org/Part:BBa_I739202 BBa_I739202].
- Digest [http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII)] in [http://partsregistry.org/Part:BBa_I739202 BBa_I739202] with SpeI/PstI.
- Digest [http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI)] in [http://partsregistry.org/Part:BBa_I739202 BBa_I739202] with SpeI/PstI.
- Ligate digested [http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII)] in [http://partsregistry.org/Part:BBa_I739202 BBa_I739202] with digested [http://partsregistry.org/Part:BBa_E0430 BBa_E0430(EYFP)]. You get a plasmid containing a [http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII)].[http://partsregistry.org/Part:BBa_E0430 BBa_E0430(EYFP)] composite part.
- Ligate digested [http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI)] in [http://partsregistry.org/Part:BBa_I739202 BBa_I739202] with [http://partsregistry.org/Part:BBa_I739009 BBa_I739009(ECFP)]. You get a plasmid containing a [http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI)].[http://partsregistry.org/Part:BBa_I739009 BBa_I739009(ECFP)] composite part.
- Digest [http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII)].[http://partsregistry.org/Part:BBa_E0430 BBa_E0430(EYFP)] with SpeI/PstI.
- Digest [http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI)].[http://partsregistry.org/Part:BBa_I739009 BBa_I739009(ECFP)] with XbaI/PstI.
- Ligate digested [http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII)].[http://partsregistry.org/Part:BBa_E0430 BBa_E0430(EYFP)] and digested [http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI)].[http://partsregistry.org/Part:BBa_I739009 BBa_I739009(ECFP)]. You get the completed plasmid containing the [http://partsregistry.org/Part:BBa_I739004 BBa_I739004(P22 cII)].[http://partsregistry.org/Part:BBa_E0430 BBa_E0430(EYFP)].[http://partsregistry.org/Part:BBa_I739008 BBa_I739008(cI)].[http://partsregistry.org/Part:BBa_I739009 BBa_I739009(ECFP)] composite part.
Plasmid 3 ([http://partsregistry.org/Part:BBa_I739204 BBa_I739204])
- Digest [http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI)], [http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP)] and the plasmid [http://partsregistry.org/Part:BBa_I739204 BBa_I739204] with EcoRI/PstI.
- Digest [http://partsregistry.org/Part:BBa_I739007 BBa_I739007(P22 cII)] and [http://partsregistry.org/Part:BBa_I739011 BBa_I739011(GFP)] with XbaI/PstI.
- Ligate digested [http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI)] and digested plasmid [http://partsregistry.org/Part:BBa_I739204 BBa_I739204].
- Ligate digested [http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP)] and the plasmid [http://partsregistry.org/Part:BBa_I739204 BBa_I739204].
- Digest [http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI)] in [http://partsregistry.org/Part:BBa_I739204 BBa_I739204] with SpeI/PstI.
- Digest [http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP)] in [http://partsregistry.org/Part:BBa_I739204 BBa_I739204] with SpeI/PstI.
- Ligate digested [http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI)] in [http://partsregistry.org/Part:BBa_I739204 BBa_I739204] with digested [http://partsregistry.org/Part:BBa_I739007 BBa_I739007(P22 cII)]. You get a plasmid containing a [http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI)].[http://partsregistry.org/Part:BBa_I739007 BBa_I739007(P22 cII)] composite part.
- Ligate digested [http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP)] in [http://partsregistry.org/Part:BBa_I739204 BBa_I739204] with [http://partsregistry.org/Part:BBa_I739011 BBa_I739011(GFP)]. You get a plasmid containing a [http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP)].[http://partsregistry.org/Part:BBa_I739011 BBa_I739011(GFP)] composite part.
- Digest [http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI)].[http://partsregistry.org/Part:BBa_I739007 BBa_I739007(P22 cII)] with SpeI/PstI.
- Digest [http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP)].[http://partsregistry.org/Part:BBa_I739011 BBa_I739011(GFP)] with XbaI/PstI.
- Ligate digested [http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI)].[http://partsregistry.org/Part:BBa_I739007 BBa_I739007(P22 cII)] and digested [http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP)].[http://partsregistry.org/Part:BBa_I739011 BBa_I739011(GFP)]. You get the completed plasmid containing the [http://partsregistry.org/Part:BBa_I739006 BBa_I739006(cI)].[http://partsregistry.org/Part:BBa_I739007 BBa_I739007(P22 cII)].[http://partsregistry.org/Part:BBa_I739010 BBa_I739010(RFP)].[http://partsregistry.org/Part:BBa_I739011 BBa_I739011(GFP)] composite part.
References
[http://www.MolecularCloning.com [1] Sambrook J and Russel DW] "Molecular Cloning: A Laboratory Manual", Cold Spring Harbour Laboratory Press, 3rd edition, 2001
[2] Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heynecker HL and Boyer HW "Construction of useful cloning vectors", Gene 2: 95-113, 1977
[3] Watson N "A new revision of the sequence of plasmid pBR322", Gene 70: 399-403, 1988
[http://www1.qiagen.com/faq/faqview.aspx?faqid=350&SearchText=&FaqCategoryId=0&MenuItemId=0&catalog=1&ProductLineId=1000228 [4] QIAGEN FAQs]
[http://www.blackwell-synergy.com/doi/abs/10.1111/j.1365-2958.1995.tb02293.x [5] Fernández S et al.] "Activation of the transcriptional regulator XylR of Pseudomonas putida by release of repression between functional domains", Mol Microbiol 16(2):205-213, 1995]
[6] Chang ACY and Cohen SN "Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid", J Bacteriol 134: 1141-1156, 1978
[7] Rose, R.E., "The nucleotide sequence of pACYC177", Nucleic Acids Res, 16(1): 356, 1988
[http://partsregistry.org/Assembly:Standard_assembly [8] Standard Assembly Process]