NYMU Taipei/Lab Notes/2007 9 16
From 2007.igem.org
< NYMU Taipei/Lab Notes(Difference between revisions)
Lihsiangyen (Talk | contribs) (→Enzyme Digestion) |
(→2nd digestion of D-term) |
||
| (7 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | ==Enzyme Digestion== | + | ==Enzyme Digestion for CinR+HSL== |
*Enzyme digestion: CinR+HSL (with RBS) with EcoRI, SpeI | *Enzyme digestion: CinR+HSL (with RBS) with EcoRI, SpeI | ||
**CinR+HSL (1~4): 14ul | **CinR+HSL (1~4): 14ul | ||
| Line 7: | Line 7: | ||
**10x EcoRI buffer: 2ul | **10x EcoRI buffer: 2ul | ||
**Total: 20ul | **Total: 20ul | ||
| + | **37° 2h | ||
*Enzyme digestion: D-term with EcoRI | *Enzyme digestion: D-term with EcoRI | ||
** Original we need double digestion EcoRI and XbaI. | ** Original we need double digestion EcoRI and XbaI. | ||
| Line 17: | Line 18: | ||
** H2O: 6ul | ** H2O: 6ul | ||
** Total: 20ul | ** Total: 20ul | ||
| + | **37°C 2h | ||
*Enzyme digestion: D-term E with XbaI | *Enzyme digestion: D-term E with XbaI | ||
**D-term E: 30ul | **D-term E: 30ul | ||
| Line 24: | Line 26: | ||
**H2O: 8ul | **H2O: 8ul | ||
**Total: 50ul | **Total: 50ul | ||
| + | **37°C 2h | ||
| - | ==Gel separation== | + | ==Digestion check of CinR+HSL and D-term by Gel separation== |
* 1% gel, TAE 1X, 30 min, 100v | * 1% gel, TAE 1X, 30 min, 100v | ||
** 11 lanes (left most is marker) | ** 11 lanes (left most is marker) | ||
| Line 49: | Line 52: | ||
**The insert of CinR+HSL 2 is wrong. | **The insert of CinR+HSL 2 is wrong. | ||
**EcoRI digestion of D-term is incomplete, hence only the upper band is isolated for DNA extraction. (The lower band is probably the negative supercoiled form of the plasmid) | **EcoRI digestion of D-term is incomplete, hence only the upper band is isolated for DNA extraction. (The lower band is probably the negative supercoiled form of the plasmid) | ||
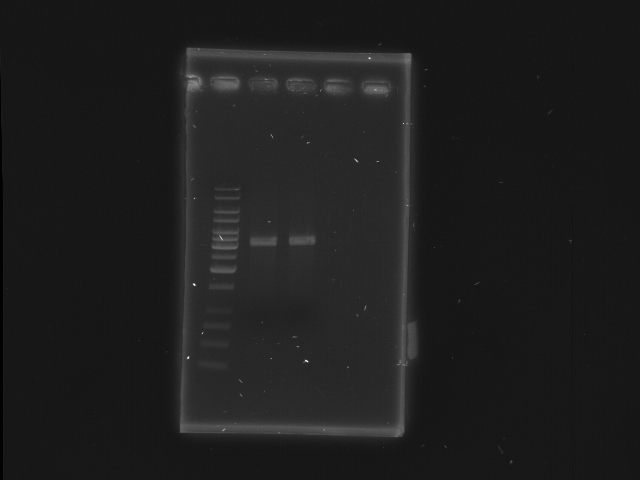
| + | == 2nd digestion of D-term and check== | ||
| + | * D-term EcoRI + XbaI | ||
| + | [[Image:NYMU_Taipei_gel_20070916_plasmid_check_2.JPG|300px]] | ||
| + | *Lane 1: 1kb ladder | ||
| + | *Lane 2, 3: D-term EcoRI, XbaI | ||
| + | *Lane 4, 5: Empty | ||
Latest revision as of 15:04, 9 October 2007
Enzyme Digestion for CinR+HSL
- Enzyme digestion: CinR+HSL (with RBS) with EcoRI, SpeI
- CinR+HSL (1~4): 14ul
- 因為濃度未知, 所以用最大體積
- EcoRI: 1ul (20,000 units/ml)
- SpeI: 1ul (10,000 units/ml)
- 10x EcoRI buffer: 2ul
- Total: 20ul
- 37° 2h
- CinR+HSL (1~4): 14ul
- Enzyme digestion: D-term with EcoRI
- Original we need double digestion EcoRI and XbaI.
- However, NEB recommend to perform the digestion in sequential manner.
- Thus, we digest the EcoR1 first
- D-term: 11ul (損失因子10, 目標重量300ng)
- 300(ng) * 10 = 3(ug) = 0.27 (ug/uL) * X(uL), X = 3/0.27 = 11.11111111
- EcoRI: 1ul
- 10x EcoRI buffer: 2ul
- H2O: 6ul
- Total: 20ul
- 37°C 2h
- Enzyme digestion: D-term E with XbaI
- D-term E: 30ul
- XbaI: 2ul
- 10x 2 buffer: 5ul
- 10x BSA: 5ul
- H2O: 8ul
- Total: 50ul
- 37°C 2h
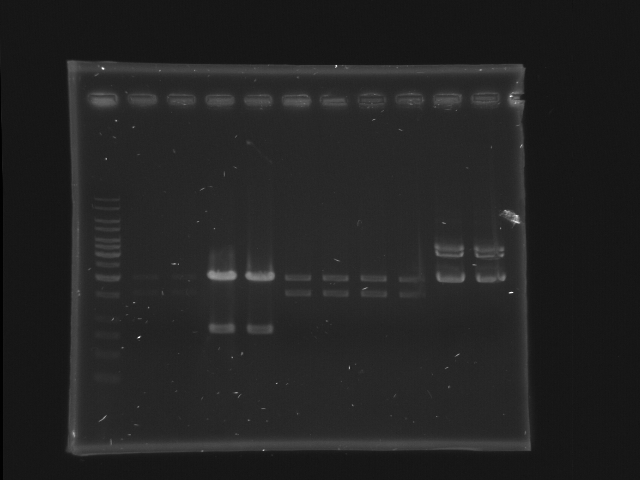
Digestion check of CinR+HSL and D-term by Gel separation
- 1% gel, TAE 1X, 30 min, 100v
- 11 lanes (left most is marker)
- each sample (24 uL) are separated into 2 lanes (12 uL for each lane)
- CinR+HSL #1-#4 (lane 2-9) and D-term (lane 10-11)
- use 1Kb ladder
- CinR+HSL insert size = 1.53 Kb
- D-term vector size = 3.284 Kb
- 6X dye
- total volume after digestion is 20uL
- thus, the dye is 4uL
- X/(20+X) = 1/6, X = 4
- sample after dye addition is 20 + 4 = 24 uL
- Lane 1: 1kb ladder (5ul)
- Lane 2, 3: CinR+HSL 1 EcoRI, SpeI
- Lane 4, 5: CinR+HSL 2 EcoRI, SpeI
- Lane 6, 7: CinR+HSL 3 EcoRI, SpeI
- Lane 8, 9: CinR+HSL 4 EcoRI, SpeI
- Lane 10, 11: D-term EcoRI
- Comments
- The concentration of CinR+HSL 1 is too low.
- The insert of CinR+HSL 2 is wrong.
- EcoRI digestion of D-term is incomplete, hence only the upper band is isolated for DNA extraction. (The lower band is probably the negative supercoiled form of the plasmid)
2nd digestion of D-term and check
- D-term EcoRI + XbaI
- Lane 1: 1kb ladder
- Lane 2, 3: D-term EcoRI, XbaI
- Lane 4, 5: Empty