Edinburgh/Yoghurt/Design
From 2007.igem.org
 Introduction | Applications | Design | Modelling | Wet Lab | References
Introduction | Applications | Design | Modelling | Wet Lab | References
Contents |
Colour Production
The zeaxanthin operon is naturally found in many plants and bacteria. The operon encodes five genes, which enable the production of the yellow pigment zeaxantin.
The five genes present in the zeaxanthin operon are:
- CrtE
- CrtB
- CrtI
- CrtY
- CrtZ
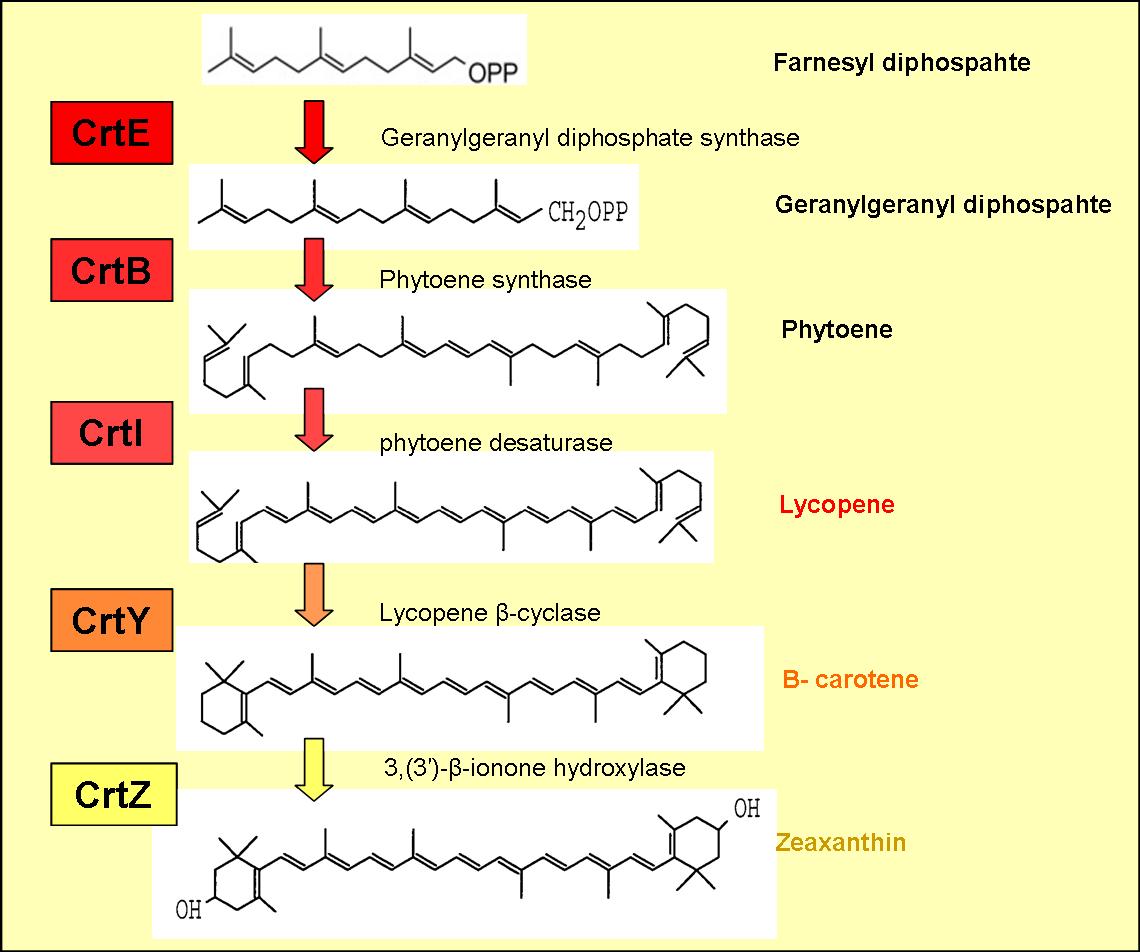
The enzymes encoded by these genes and the products formed are displayed in figure 1.
There are three pigments produced by the genes with in the zeaxanthin operon and a brief description of each is given below:
Lycopene
The genes CrtE, CrtB and CrtI produce lycopene from farnesyl diphosphate (an intermediate in the mevlonate pathway).
Lycopene is a red pigment (found in tomatoes), which also has extremally powerful antioxidant properties and may possibly help protect you from cancer.
B-carotene
B-carotene is produced by cyclising lycopene, which is carried out by lycopene B-cyclase encoded by the gene CrtY (see figure )
B-carotene has an orange pigmentation and is responsible for the colour of carrots, winter squash and several other vegetables. The pigment can be stored in the liver and converted to Vitamin A, a form of retinol, required for sight.
zeaxanthin
Addition of CrtZ to the CrtEBIY construct enables the hydroxylation of B-carotene (see figure 1) to zeaxanthin, which is a yellow pigment.
Proposed Biobricks
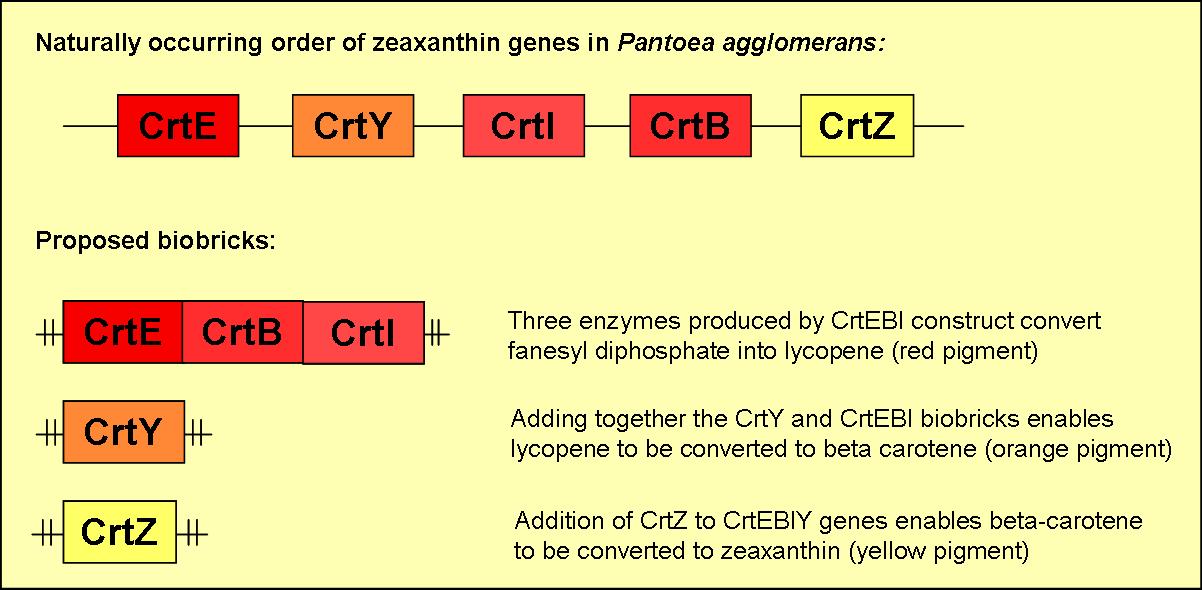
We plan to produce five different biobricks with varying combinations of the five zeaxanthin genes. A brief overview of three of these biobricks can be found in figure 2.
Biobrick 1
- will consist of the three genes CrtE, CrtB & CrtI
- will produce the red pigment lycopene
Biobrick 2
- will contain CrtY
- when induced in the presence of functional CrtEBI genes an orange pigment (B-carotene) will be formed
Biobrick 3
- will consist of CrtZ
- when induced in the presence of functional CrtEBIY genes it will enable the formation of the yellow pigment zeaxanthin
Biobrick 4
- will contain the four genes CrtE, CrtB, CrtI & CrtY
- the construct will be capable of producing B-carotene
Biobrick 5
- will contain all five genes present in the zeaxanthin operon; CrtE, CrtB, CrtI, CrtY & CrtZ
- biobrick will enable the production of zeaxanthin (yellow pigmentation)
Vanilla Flavour Production
In order to create vanilla flavouring for our yoghurt, we have designed a novel vanillin biosynthesis pathway. This pathway consists of five different genes, which enable the synthesis of vanillin from the amino acid tyrosine.
We chose to use tyrosine as a starting point, as the amino acid is produced endogenously by bacteria, and is also present within milk proteins. Theoretically this means a starting substrate will not have to be added to the yoghurt starter culture, to faciliate the synthesis of vanillin.
The vanillin biosynthesis pathway was created by piecing together five different genes from three completely different organisms. These five genes are:
- Sam8
- Sam5
- COMT
- Fcs
- Ech
Sam8
- isolated from the bacterium Saccharothrix espeanensis
- encodes tyrosine ammonia lyase (catalyses the deamination of tyrosines amine group)
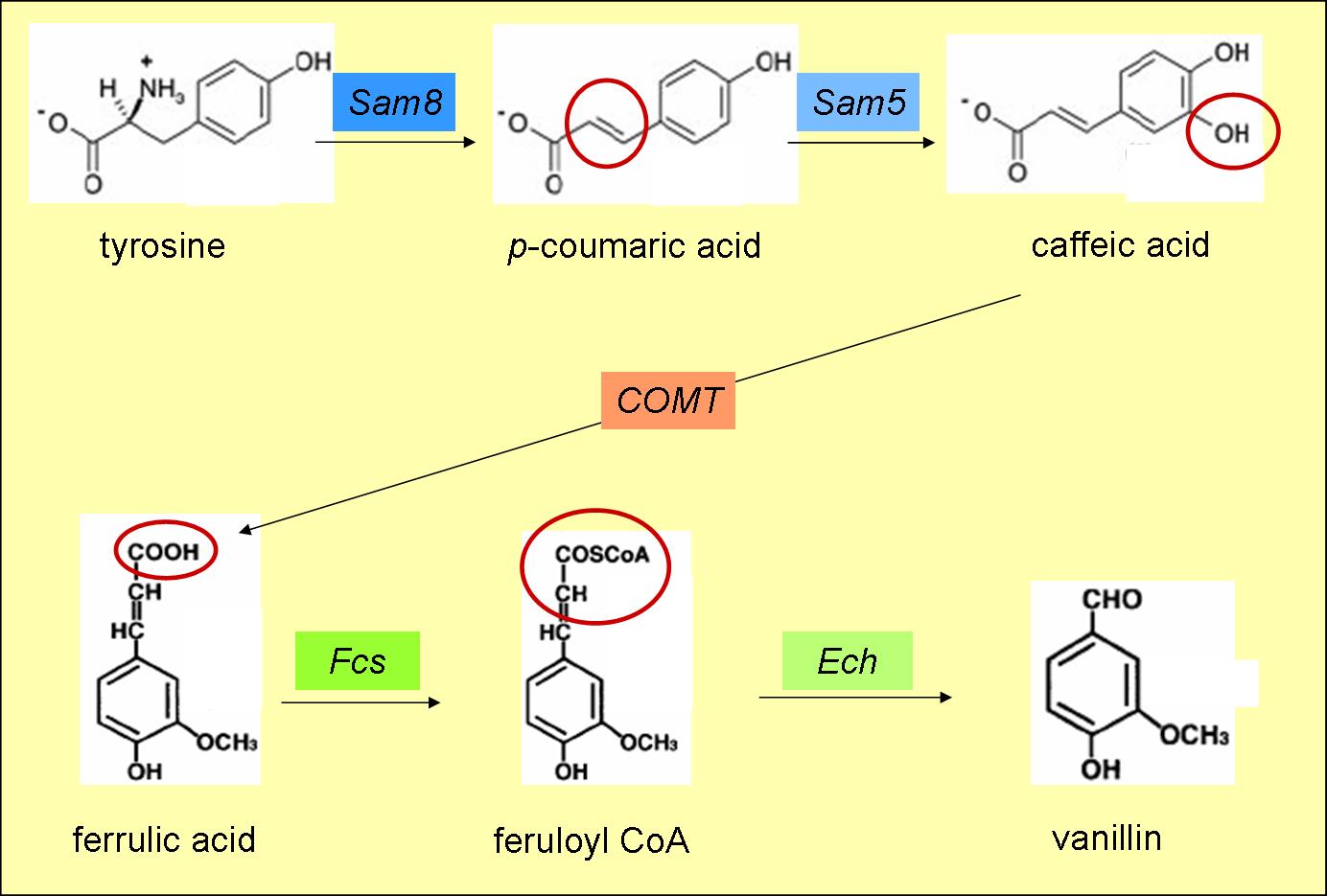
- converts tyrosine to p-coumaric acid (fig. 3)
- catalyses the first step in the vanillin biosynthesis pathway
Sam5
- also isolated from Saccharothrix espeanensis
- encodes 4-coumarate 3-hydroxylase (hydroxylates C4 in the aromatic ring of p-coumaric acid)
- converts p-coumaric acid to caffeic acid (fig. 3)
- catalyses the second step in the vanillin biosynthesis pathway
COMT
- enzyme is natively found in the plant alfalfa
- encodes caffeic acid-O-methyl transferase (methylates -OH on C4 of the aromatic ring)
- produces ferulic acid from caffeic acid (fig. 3)
- catalyses the third step of the vanillin biosynthesis pathway
Fcs
- gene was isolated from Pseudomonas fluorescens
- encodes feruoyl CoA synthase (ligates acetyl-CoA onto ferulic acid)
- produces feruloyl CoA from ferrulic acid (fig. 3)
- catalyses the penultimate of the vanillin biosynthesis pathway
Ech
- gene was also isolated from Pseudomonas fluorescens
- encodes enoyl CoA hydratase (cleaves CoA group from feruloyl CoA)
- converts feruloyl CoA to vanillin (fig. 3)
- catalyses the final stage of the vanillin biosynthesis pathway
Proposed Biobricks
We plan to construct the vanillin pathway in three separate constructs:
Construct 1
- consist of Sam5 and Sam8 genes ligated together with ribosome binding sites
- we will test the construct for the production of caffeic acid from tyrosine
Construct 2
- will only contain the COMT gene ordered from GENEART, together with ribosome binding site
- this construct will not be tested on its own for acitivty
Construct 3
- will contain the ech and fcs genes along with ribosome binding sites
- two genes will be tested for the formation of vanillin from ferulic acid
Lemon Flavour Production
Gene Expression In Lactobacillus
In order to express our flavour and colour genes in lactic acid bactia (LAB), we require a different vector to those currently in use by the registry. After some research, we found that a few groups withint the UK were working with LAB.
We kindly thank Dr Mike Gasson & Dr. Claire Shearman, of the Institute of Food Research in Norwich for generously donating the pTG262 vector.
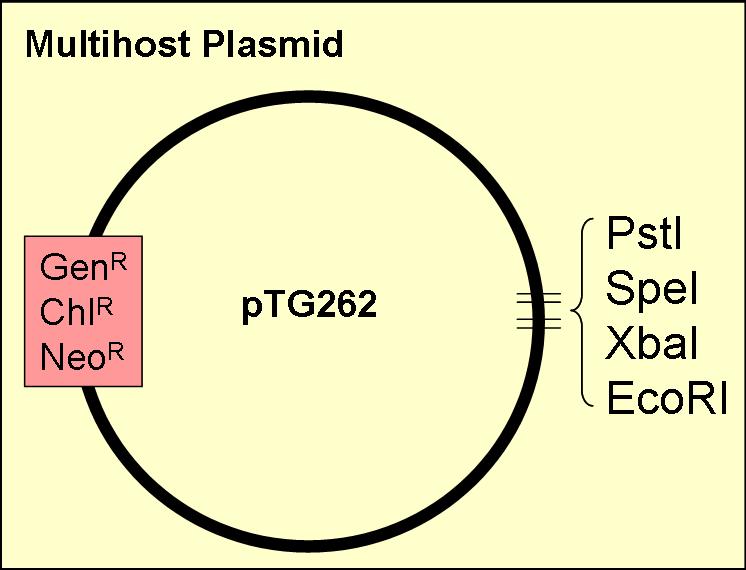
pTG262
This vector was ideal for use as a biobrick chassey, as:
- it contained three of the four biobrick restriction enzyme sites (insertion of a biobrick added the other two)
- it has a gram positive origin of replication, which works well in E. coli (enabling the plasmid to act as a shuttle vector between E. coli and LAB
- contains three antibiotic resistance sites (chloramphenicol, neomycin & gentamicin)
- known to work in Lactobacillus, Lactococcus and Bacillus as well as E. coli
We also plan to test the efficieny of the vector in other gram negative bacteria including; Shewanella & Pseudomonas, Agrobacterium
Introduction | Applications | Design | Modelling | Wet Lab | References