Edinburgh/Yoghurt/Proof of concept
From 2007.igem.org
(→pTG262-Pbad-RFP construct) |
|||
| Line 4: | Line 4: | ||
In order to test the feasibility of gene expression in ''Lactobacillus'' and the possibility of making self flavouring yoghurt, we required a proof of concept. | In order to test the feasibility of gene expression in ''Lactobacillus'' and the possibility of making self flavouring yoghurt, we required a proof of concept. | ||
| - | As both the pigment and flavour pathways are rather complex and will require modification and optimisation before their expression in yoghurt we are going to use the much simpler RFP gene as a proof of concept. | + | As both the pigment and flavour pathways are rather complex and will require modification and optimisation before their expression in yoghurt we are going to use the much simpler Red Fluorescent Protein (RFP) gene as a proof of concept. |
So far we have managed to transform a number of RFP-pTG262 vectors containing a variety of promoters into ''Bacillus subtillis'', a gram positive bacterium. | So far we have managed to transform a number of RFP-pTG262 vectors containing a variety of promoters into ''Bacillus subtillis'', a gram positive bacterium. | ||
| Line 15: | Line 15: | ||
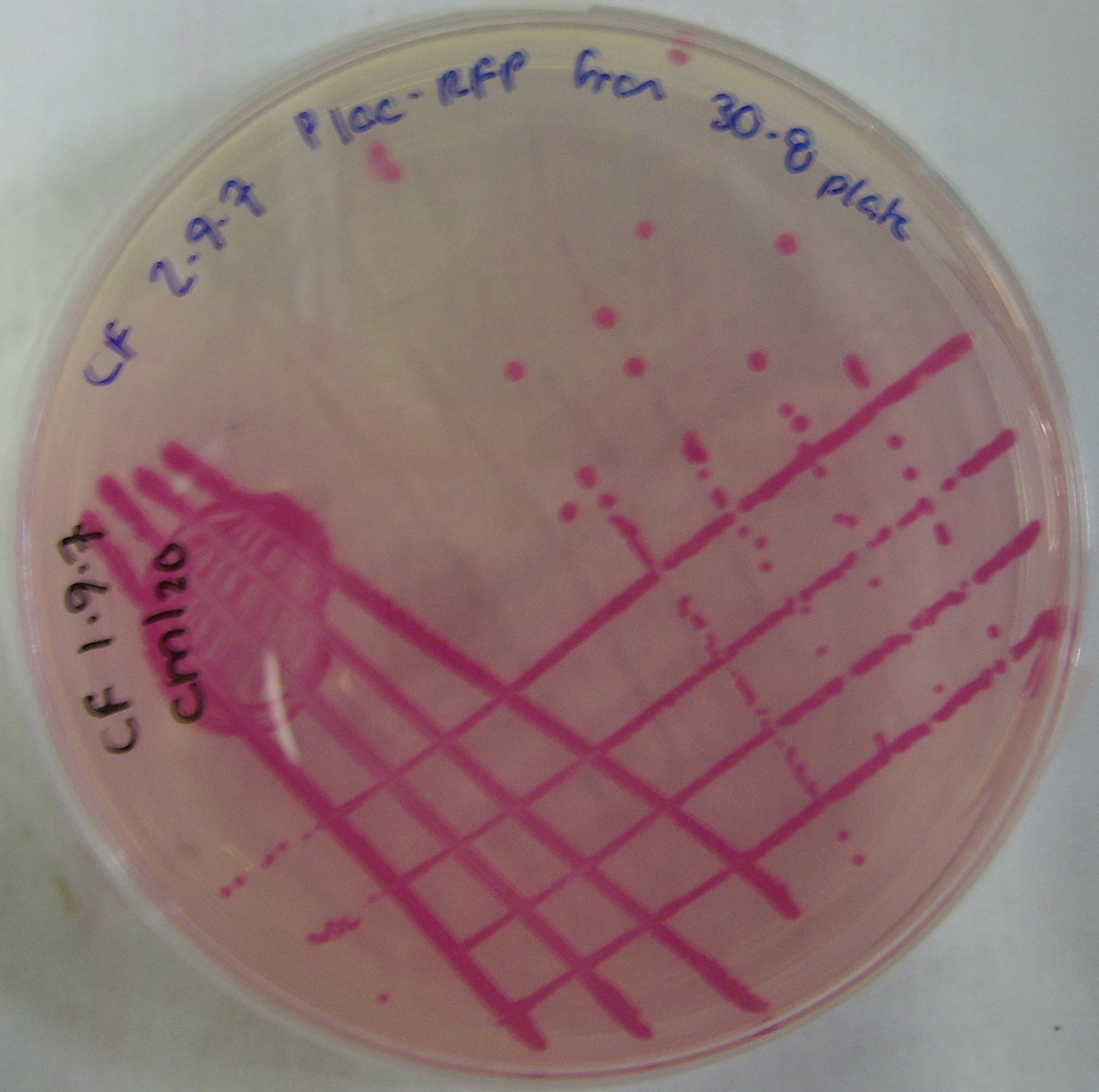
====pTG262-PLac-RFP construct==== | ====pTG262-PLac-RFP construct==== | ||
| + | |||
| + | [[Image:PLac-RFP-pTG262 Bacillus.JPG|thumb|200 px|'''Fig. 1:''' ''E. coli'' colonies successfully transformed with the pTG262-pLac-RFP construct]] | ||
| + | |||
We have inserted the lactose induced RFP gene into the pTG262 vector | We have inserted the lactose induced RFP gene into the pTG262 vector | ||
* this vector was then transformed into E. coli | * this vector was then transformed into E. coli | ||
| - | * growth on IPTG-Xgal media resulted in RFP expression and production of red colonies | + | * growth on IPTG-Xgal media resulted in RFP expression and production of red colonies, these can be viewed in figure 1 |
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
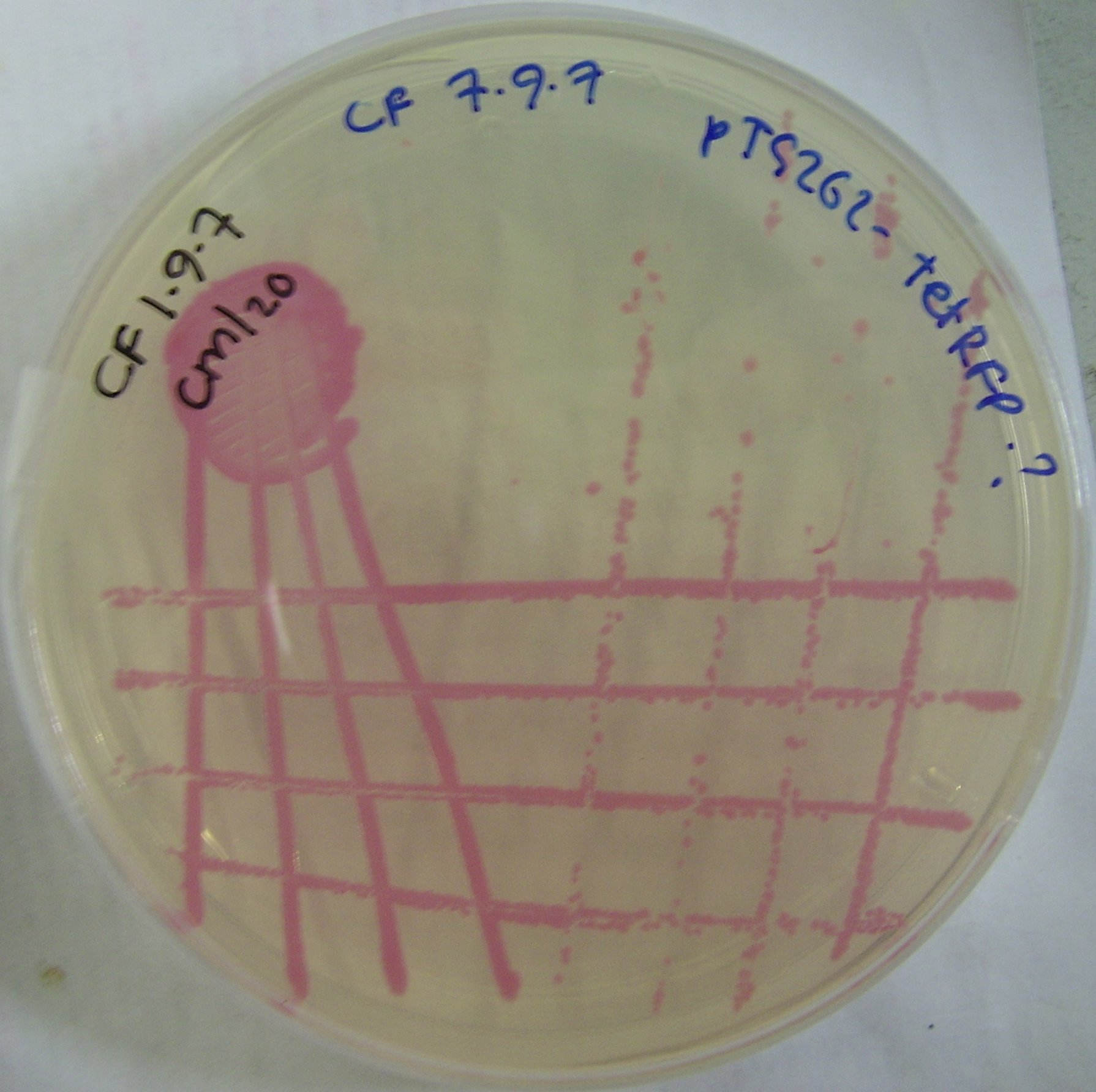
| - | + | ====pTG262-Ptet-RFP construct==== | |
| + | |||
| + | |||
| + | [[Image:PTet-RFP-pTG262 Bacillus.JPG|thumb|left|'''Fig. 2:''' ''E. coli'' colonies successfully transformed with pTG262-Ptet-RFP construct|200 px]] | ||
| + | |||
| + | |||
| + | |||
| + | We ligated the Ptet induced RFP biobrick into the pTG262 vector. We then transformed the vector into ''E. coli''. | ||
| + | |||
| + | RFP synthesis was observed when ''E. coli'' colonies containing the vector were incubated on tetracycline containing plates. | ||
| + | |||
| + | ptet incuded RFP ''E. coli'' colonies can be viewed in figure 2. | ||
| + | |||
| + | |||
| + | |||
| + | ====pTG262 expression in ''Bacillus subtilis''==== | ||
| + | |||
| + | After the successful expression of both the pTG262 RFP constructs in ''E. coli'', we decided to determine if pTG262 could be stably experessed in ''Bacillus subtilis''. | ||
| + | |||
| + | To do this we transformed both the pTG262 and Plac-RFP-pTG262 vectors into ''Bacillus subtilis''. | ||
| + | |||
| + | Figure 3 depicts the successful transformation and expression of pTG262 in ''Bacillus subtilis''. | ||
| + | |||
| + | This is determined by the ability of ''Bacillus'' to grow on chloramphenicol plates. | ||
| + | |||
| + | Unfortunately RFP was not expressed from the pLac promoter, as shown by the colonies being white not red. We are attributing this to the ''Bacillus'' transcription machinary (particulally RNA polymersase and its associated sigma factors) not recognising the pLac promoter. | ||
| + | |||
| + | |||
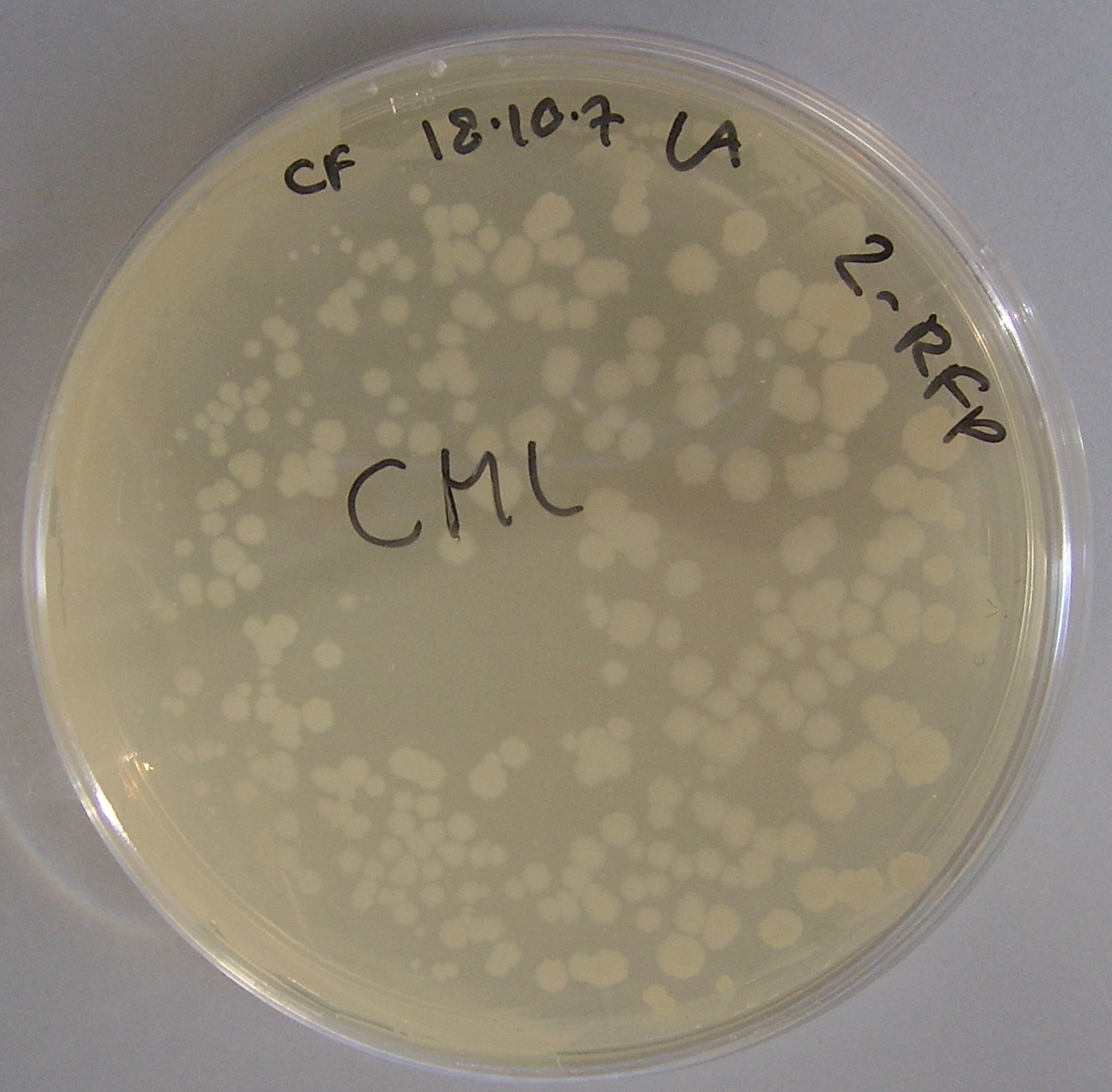
| + | We also transformed the a lacz-pTG262 vector into ''Bacillus subtilis''. | ||
| + | |||
| + | When the transformants were grown on an IPTG containing media colonies turned blue, indicating that lacz (beta-galactosidase gene) was successfully expressed in ''Bacillus subtilis''. | ||
| + | |||
| + | The results of this experiment may be viewed in figure 4. | ||
| + | |||
| + | |||
| + | [[Image:PTG262 Bacillus colonies.JPG|thumb|'''Fig. 3:''' ''Bacillus subtillis'' colonies successfully transformed with pTG262-plac-RFP vector|200 px]] | ||
| + | |||
'''Transforming ''Bacillus subtillis'' with pTG262 vector''' | '''Transforming ''Bacillus subtillis'' with pTG262 vector''' | ||
| - | |||
* we have managed to transform the pTG262 vector into ''Bacillus subtillis'' sucessfully | * we have managed to transform the pTG262 vector into ''Bacillus subtillis'' sucessfully | ||
* colonies of ''Bacillus'' growing on chloramphenicol plates can be viewed in Fig 1. | * colonies of ''Bacillus'' growing on chloramphenicol plates can be viewed in Fig 1. | ||
| - | |||
| - | + | ||
Revision as of 17:42, 25 October 2007
 Introduction | Applications | Design | Modelling | Wet Lab | Proof of concept | References
Introduction | Applications | Design | Modelling | Wet Lab | Proof of concept | References
In order to test the feasibility of gene expression in Lactobacillus and the possibility of making self flavouring yoghurt, we required a proof of concept.
As both the pigment and flavour pathways are rather complex and will require modification and optimisation before their expression in yoghurt we are going to use the much simpler Red Fluorescent Protein (RFP) gene as a proof of concept.
So far we have managed to transform a number of RFP-pTG262 vectors containing a variety of promoters into Bacillus subtillis, a gram positive bacterium.
We chose to initially transform Bacillus subtillis with our proof of concept vectors over Lactobacillus for two reasons:
1. the bacterium has a much simpler transformation process
2. members of the French lab have had successfull results from when they previously worked with Bacillus subtilis
pTG262-PLac-RFP construct
We have inserted the lactose induced RFP gene into the pTG262 vector
- this vector was then transformed into E. coli
- growth on IPTG-Xgal media resulted in RFP expression and production of red colonies, these can be viewed in figure 1
pTG262-Ptet-RFP construct
We ligated the Ptet induced RFP biobrick into the pTG262 vector. We then transformed the vector into E. coli.
RFP synthesis was observed when E. coli colonies containing the vector were incubated on tetracycline containing plates.
ptet incuded RFP E. coli colonies can be viewed in figure 2.
pTG262 expression in Bacillus subtilis
After the successful expression of both the pTG262 RFP constructs in E. coli, we decided to determine if pTG262 could be stably experessed in Bacillus subtilis.
To do this we transformed both the pTG262 and Plac-RFP-pTG262 vectors into Bacillus subtilis.
Figure 3 depicts the successful transformation and expression of pTG262 in Bacillus subtilis.
This is determined by the ability of Bacillus to grow on chloramphenicol plates.
Unfortunately RFP was not expressed from the pLac promoter, as shown by the colonies being white not red. We are attributing this to the Bacillus transcription machinary (particulally RNA polymersase and its associated sigma factors) not recognising the pLac promoter.
We also transformed the a lacz-pTG262 vector into Bacillus subtilis.
When the transformants were grown on an IPTG containing media colonies turned blue, indicating that lacz (beta-galactosidase gene) was successfully expressed in Bacillus subtilis.
The results of this experiment may be viewed in figure 4.
Transforming Bacillus subtillis with pTG262 vector
- we have managed to transform the pTG262 vector into Bacillus subtillis sucessfully
- colonies of Bacillus growing on chloramphenicol plates can be viewed in Fig 1.
Introduction | Applications | Design | Modelling | Wet Lab | Proof of concept | References