Paris/July 4
From 2007.igem.org
Nicolas C. (Talk | contribs) |
(→Measuring excretion of Dap by MG1655 DapA+ strain in an indirect manner) |
||
| (13 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | [[Paris/July 3|yesterday]] -- [[Paris/July 5|tomorrow]] | |
| - | + | == Transduction to MG1655 using the P1 stock made on w121.== | |
| - | + | See [[Paris/PROTOCOLS|protocols]]. | |
| - | + | ||
| - | [[ | + | |
| + | == Growth kinetics : Results of July, 3 == | ||
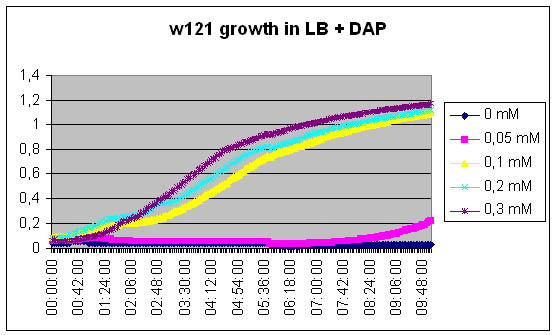
| + | === Growth of DapA- strain (w121) relative to the concentration of DAP in the medium === | ||
| + | [[Image:W121 LB-DAP.jpg]] | ||
| + | === Measuring excretion of Dap by MG1655 DapA+ strain in an indirect manner === | ||
| + | [[Image:W121 S0-DAP.jpg]]<br> | ||
| + | The w121 did not grow at all in the S0 medium... Either we did something wrong, or there is a logical explanation ! There might be growth inhibitors in the ON medium, their might be no nutriments left in the medium...<br> | ||
We will redo the experiment but this time, the medium will be recovered from exponential phase culture of MG1655 | We will redo the experiment but this time, the medium will be recovered from exponential phase culture of MG1655 | ||
| - | [[Paris|<<home]] | + | == Acinetobacter liquid culture == |
| + | |||
| + | We picked up a colony from LB solid culture from 07/03/2007 and put it into 5mL LB medium (30°C aerobic) (overnight culture). | ||
| + | |||
| + | == Low Nitrogen Minimum Medium with Nile Red == | ||
| + | |||
| + | This medium allows Acinetobacter ADP1 to synthesize triglyceride and wax ester. Lipid inclusions are seen by fluorescent dye (Nile Red). | ||
| + | |||
| + | See [[Paris/PROTOCOLS#Low_Nitrogen_Minimum_Medium|protocols]]. | ||
| + | |||
| + | == pKS::DGAT Plasmid: transformation == | ||
| + | |||
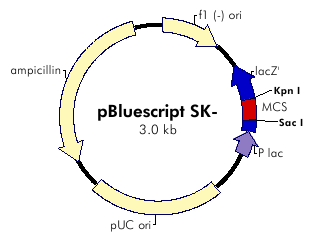
| + | The plasmid contains ampicilline resistance gene and pLac promotor upstream the transgene DGAT (Diacylglycerol Acyltransferase). | ||
| + | |||
| + | [[Image: Pbluescript_SK-.jpg|center]] | ||
| + | |||
| + | We cloned the plasmid by bacterial transformation (Subcloning Efficiency DH5alpha Competent Cells: See [[Paris/PROTOCOLS#Chemical_transformation|protocols]]). | ||
| + | |||
| + | [[Paris|<<home]]--[[Paris/July 3|yesterday]] -- [[Paris/July 5|tomorrow]] | ||
Latest revision as of 09:43, 31 August 2007
Contents |
Transduction to MG1655 using the P1 stock made on w121.
See protocols.
Growth kinetics : Results of July, 3
Growth of DapA- strain (w121) relative to the concentration of DAP in the medium
Measuring excretion of Dap by MG1655 DapA+ strain in an indirect manner
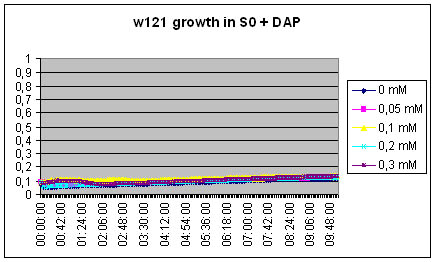
The w121 did not grow at all in the S0 medium... Either we did something wrong, or there is a logical explanation ! There might be growth inhibitors in the ON medium, their might be no nutriments left in the medium...
We will redo the experiment but this time, the medium will be recovered from exponential phase culture of MG1655
Acinetobacter liquid culture
We picked up a colony from LB solid culture from 07/03/2007 and put it into 5mL LB medium (30°C aerobic) (overnight culture).
Low Nitrogen Minimum Medium with Nile Red
This medium allows Acinetobacter ADP1 to synthesize triglyceride and wax ester. Lipid inclusions are seen by fluorescent dye (Nile Red).
See protocols.
pKS::DGAT Plasmid: transformation
The plasmid contains ampicilline resistance gene and pLac promotor upstream the transgene DGAT (Diacylglycerol Acyltransferase).
We cloned the plasmid by bacterial transformation (Subcloning Efficiency DH5alpha Competent Cells: See protocols).