Tianjin/FLIP-FLOP
From 2007.igem.org
| Line 51: | Line 51: | ||
==<font size="4" color="#0000CC">Experiment</font>== | ==<font size="4" color="#0000CC">Experiment</font>== | ||
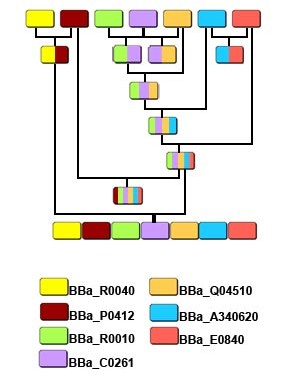
| - | <font size="3" color="#0099CC">'''1.'''</font><font size="3" color="#0099CC">'''Ligation Strategy'''</font> | + | <font size="3" color="#0099CC">'''1.'''</font><font size="3" color="#0099CC">'''Ligation Strategy'''</font><[[Tianjin/FLIP-FLOP/More details|More details]]> |
<br> | <br> | ||
<table width="99%" border="0" cellpadding="0" cellspacing="10" style="padding: 10px; background-color: #FFFF99"> | <table width="99%" border="0" cellpadding="0" cellspacing="10" style="padding: 10px; background-color: #FFFF99"> | ||
| Line 57: | Line 57: | ||
<td><center>[[Image:TJUzmlligation.jpg|300px]] </td> | <td><center>[[Image:TJUzmlligation.jpg|300px]] </td> | ||
<td><div id="Experiment"> | <td><div id="Experiment"> | ||
| - | In order to assemble our device genetically in the shortest period, we try different method-Rolling Assembly and Parallel Assembly at the same time. | + | In order to assemble our device genetically in the shortest period, we try different method-Rolling Assembly and Parallel Assembly at the same time. |
<ul> | <ul> | ||
<li>E.coli Cultivate</li> | <li>E.coli Cultivate</li> | ||
Revision as of 17:35, 26 October 2007
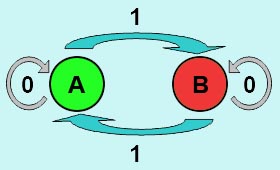
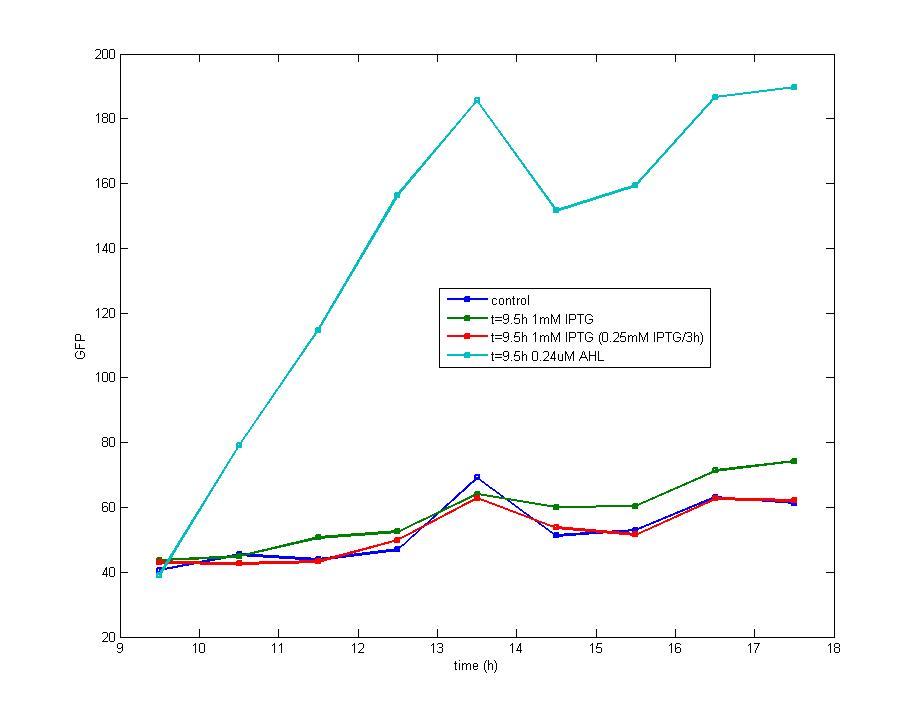
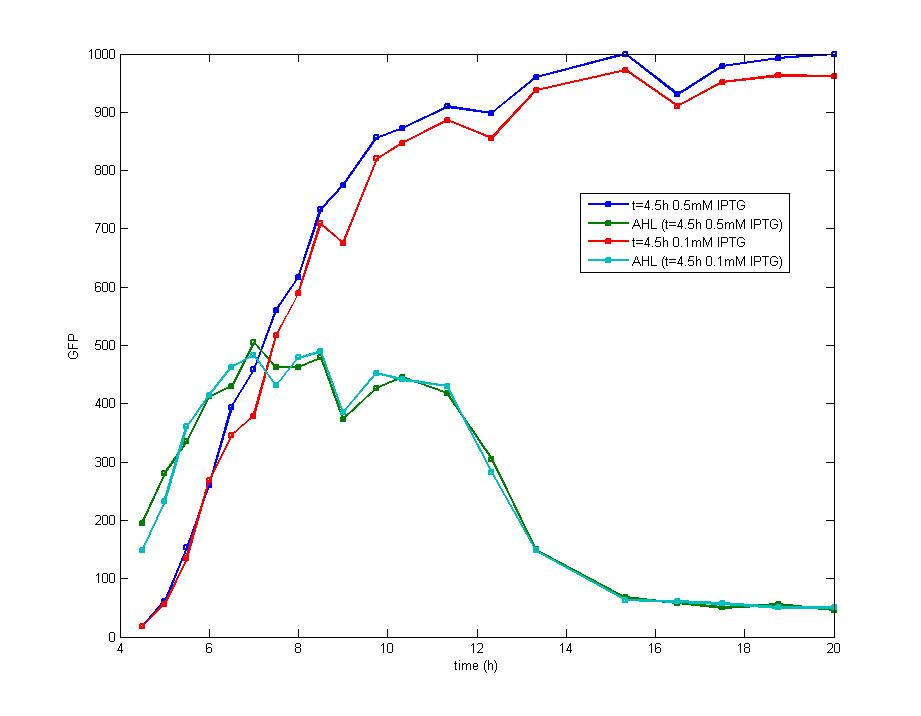
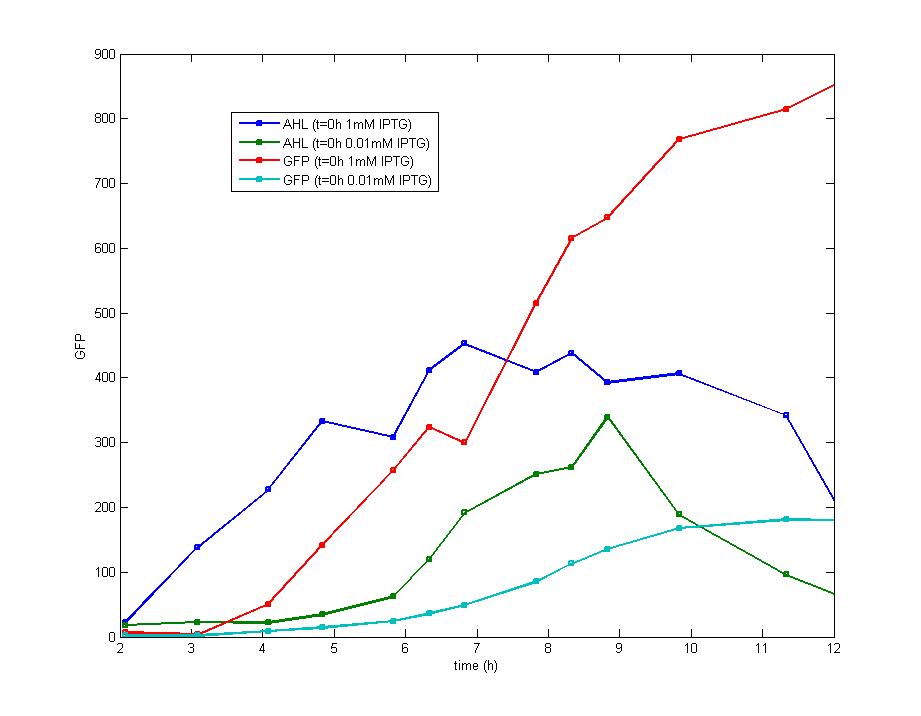
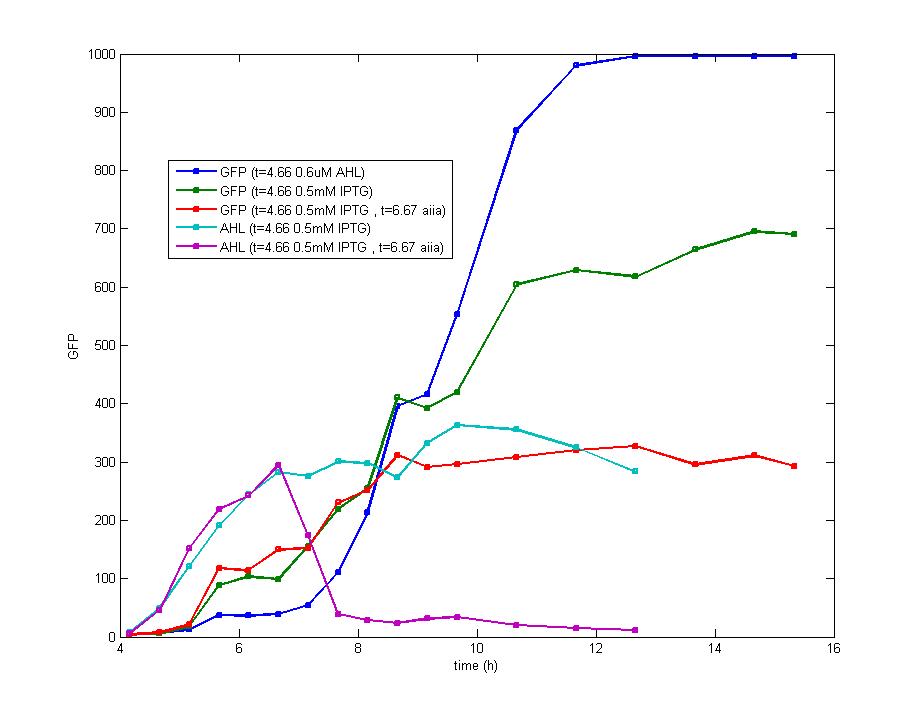
DesignFlip-Flop, a huge family of basic electric elements, is widely applied to the field of electric circuit and construction of database. One of the most common members of this family is RS Flip-Flop, the key part of which is a clock-controlled element. Based on the conception of "Flip-flop" and synthetic biology, we designed the Genetically RS FLIP-FLOP whose output signal(Green Fluorescence) is regulated by additional input signal (the addition of IPTG). Besides this, we modulate the performance of Genetically RS FLIP-FLOP to optimize our original design. 1.Introduction to the logic rules of our flip-flop
The logic principle of our design is shown above. Unless the input signal transfer from one stable condition to another (such as 0 to 1 or 1 to 0), the output signal would change into 1, otherwise it would maintain 0. Thus, the immediate response to emergency (input change) enables our design to detect signal variation in a short time, which is beneficial for process control because of its time-saving character.
| |||
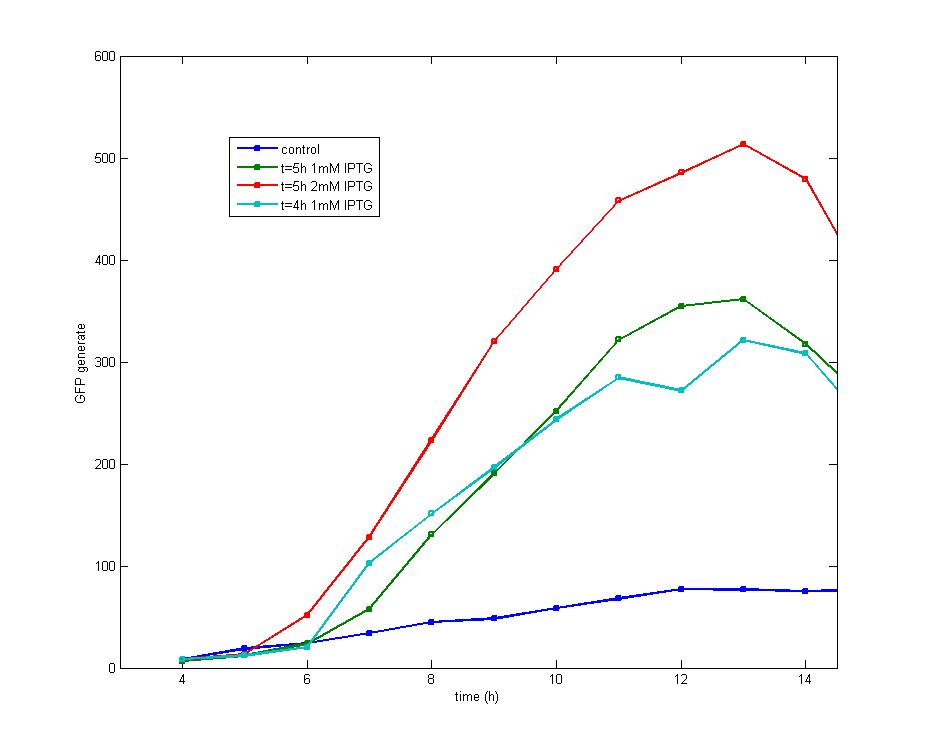
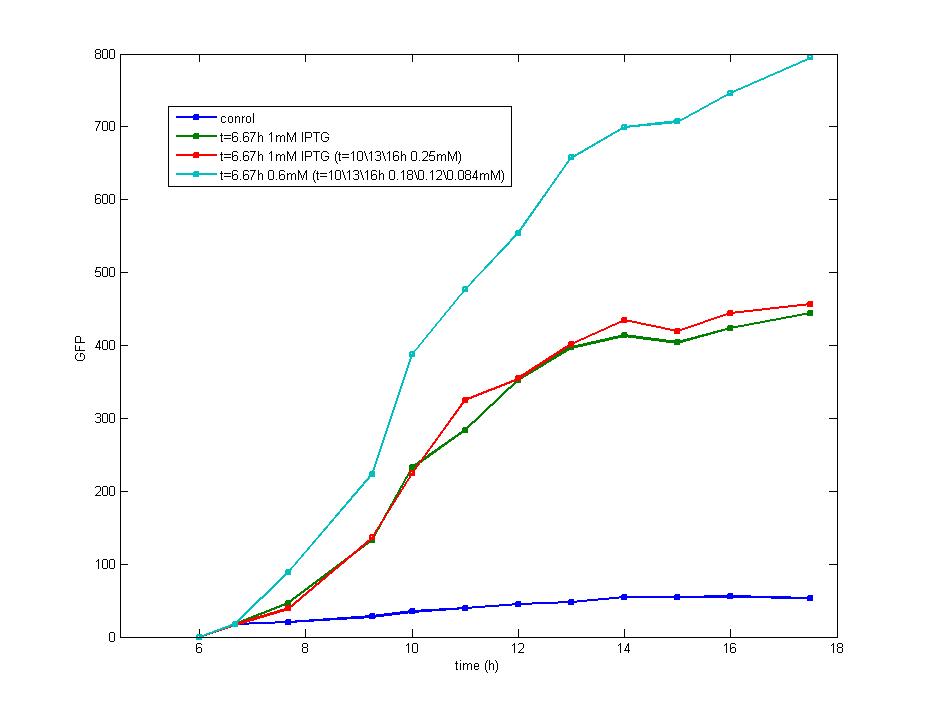
ModelingBased on Ordinary Differential Equations, we construct the Mathematical Model of our flip-flop system to test the result of our design and predict potential key factors deciding the results of our experiment. According to the model, the variation of output signal responding to the input signal matches the typical feature of flip-flop, there would no output signal only at the positive edge and negative edge of the input signal. The influence of AHL to the output signal is also considered. By changing the degradation speed of AHL, the fluorescence intensity exhibit different behaviour which could be explained by principles of flip-flop. Finally, the parameter sensitivity is also tested to explore most significant parameter to output signals and it is discovered that the strength of promoter I, which controls the production of LuxI, the promoter II, which controls the expression of LuxR, exert great effect on the final results. 1. Construction of Mathematical Model
| |||
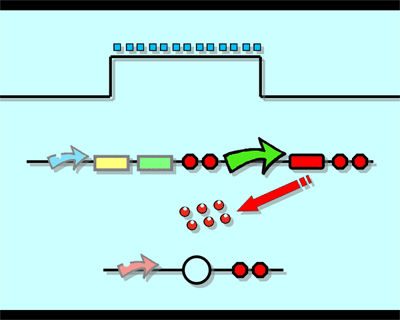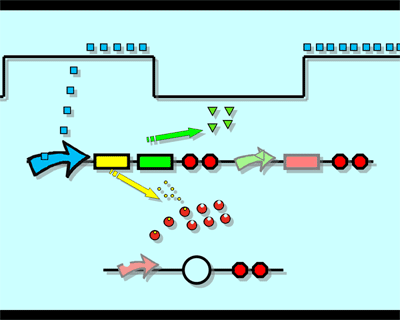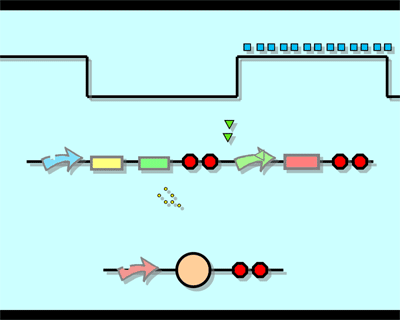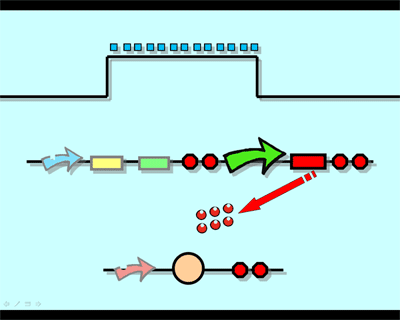
Experiment1.Ligation Strategy<More details>
2.
Parts Reservoir
|