Tianjin/FLIP-FLOP/Experiment/result
From 2007.igem.org
Sunlovedie (Talk | contribs) |
Lovecarrot (Talk | contribs) |
||
| (17 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{| | {| | ||
| - | + | |- | |
| - | [[Image:tjuexpff201.jpg| | + | |width="960px" style="padding: 10px; background-color: #FFFF99" | |
| - | After the addition of 1 mM IPTG into cultures with an | + | <table width="99%" cellpadding="0" cellspacing="10" style="padding: 10px; background-color: #FFFF99"> |
| - | + | <tr> | |
| - | + | <td>[[Image:tjuexpff201.jpg|300px]]</td> | |
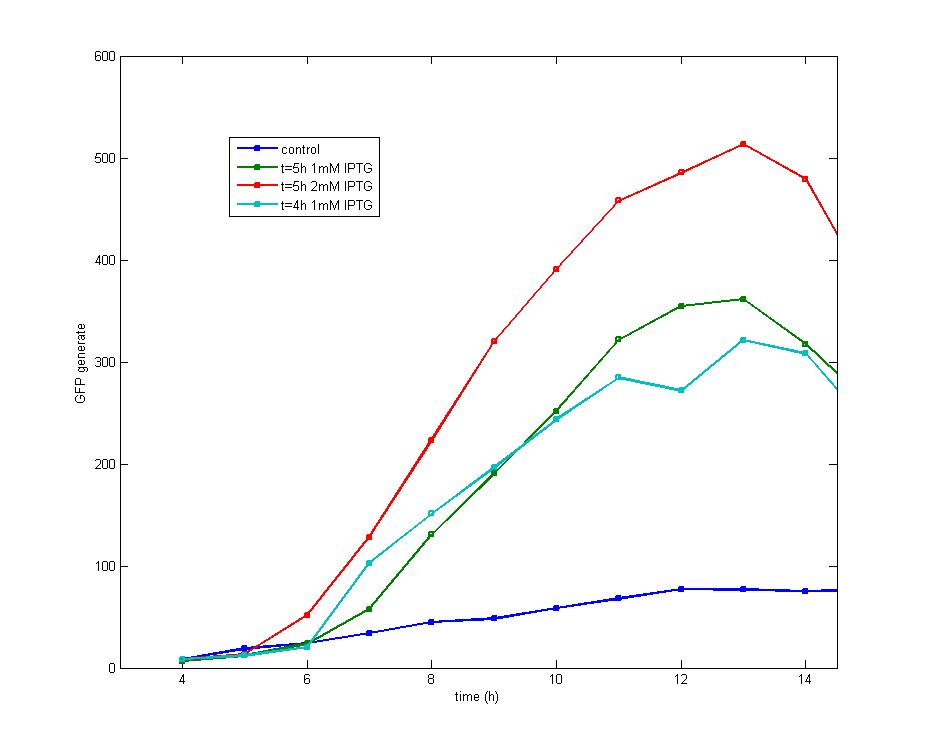
| - | The supplement of IPTG exerts little effect on the shape of curves | + | <td>Figure 1. After the addition of 1 mM IPTG into cultures with an OD<sub>600</sub> value of 0.4(cultured for 4 hours) and 0.6(cultured for 5 hours) respectively, it is discovered that both share the similar shape of GFP peak while early addition of IPTG could produce early peak. Addition of 2mM IPTG tends to produce a higher peak than that of 1mM IPTG at the OD<sub>600</sub>=0.6, but both curves share the same changing tendency.</td> |
| - | The fluorescence intensity tends to increase constantly with addition of AHL instead of IPTG, as the blue line shows, while under the latter condition it becomes steady and has a lower value than that with addition of AHL, proving that the | + | </tr></table> |
| - | + | <table width="99%" cellpadding="0" cellspacing="10" style="padding: 10px; background-color: #FFFF99"> | |
| - | [[Image:tjuexpff203.jpg| | + | <tr> |
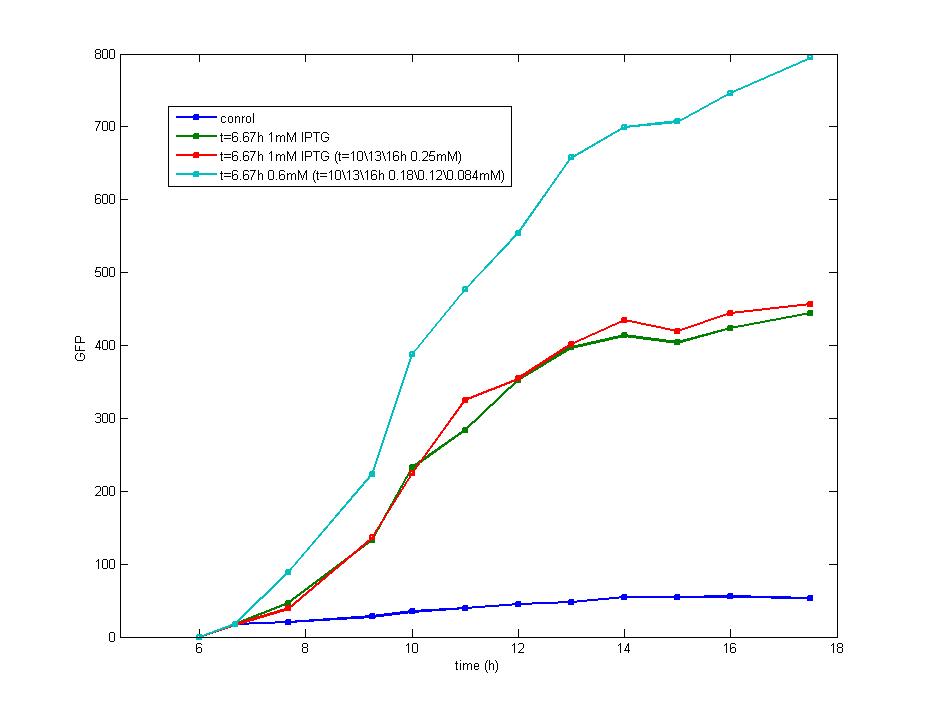
| - | The impulse of IPTG at the stationary phase could not stimulate the production of fluorescence protein which is shown in this graph. Considering the addition of AHL could also result in the accumulation of fluorescence protein which | + | <td>Figure 2. The supplement of IPTG exerts little effect on the shape of curves which indicates that the induction of IPTG mainly occurs at the beginning of impulse and keeps stable since then on.The fluorescence intensity tends to increase constantly with addition of AHL instead of IPTG, as the blue line shows, while under the latter condition it becomes steady and has a lower value than that with addition of AHL, proving that the addition of IPTG triggers the inhibition of LuxR Protein expression.</td> |
| - | + | <td>[[Image:tjuexpff202.jpg|300px]]</td> | |
| - | + | </tr></table> | |
| - | The concentration of extracellular AHL is examined after the induction of diluted IPTG and it seems there is no difference between the | + | |
| - | + | <table width="99%" cellpadding="0" cellspacing="10" style="padding: 10px; background-color: #FFFF99"> | |
| - | [[Image:tjuexpff205.jpg| | + | <tr> |
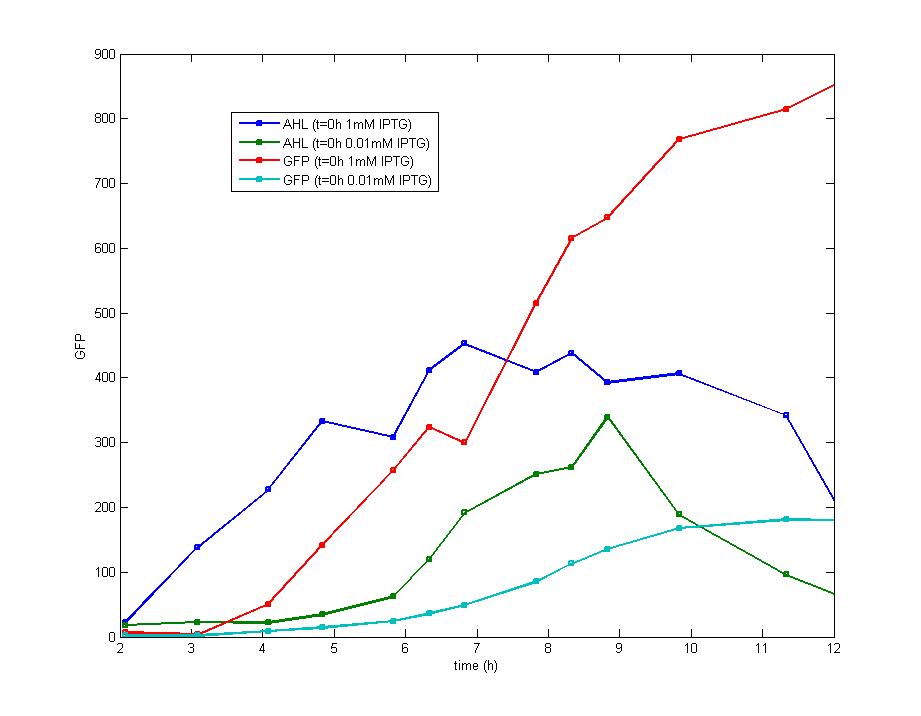
| - | The addition of IPTG with a concentration of 1 mM and 0.1 mM | + | <td>[[Image:tjuexpff203.jpg|300px]]</td> |
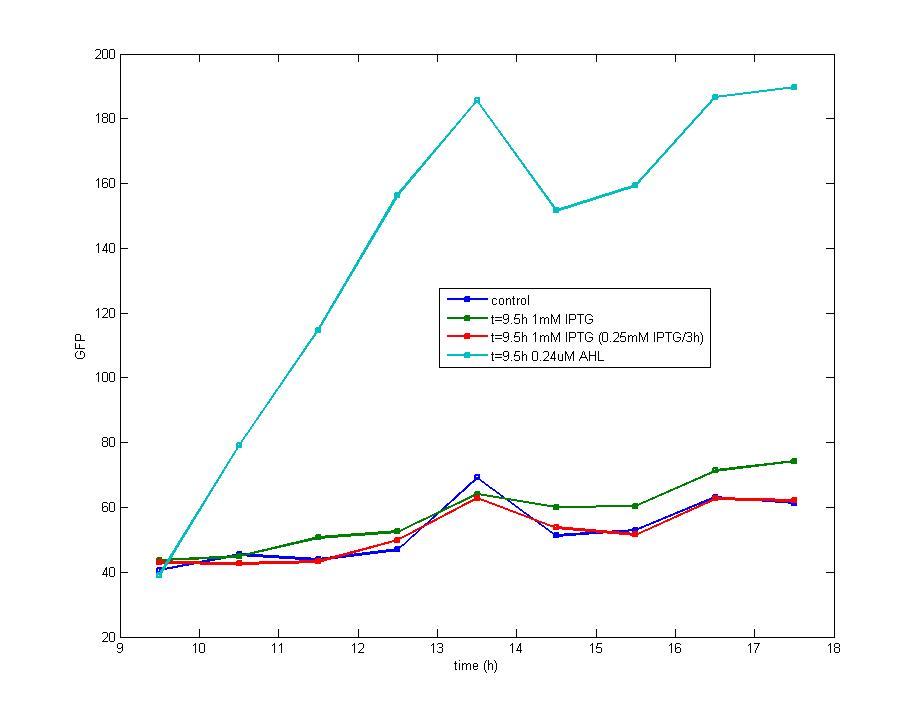
| - | < | + | <td>Figure 3. The impulse of IPTG at the stationary phase could not stimulate the production of fluorescence protein, which is shown in this graph. Considering the addition of AHL could also result in the accumulation of fluorescence protein, which supports the existence of LuxR protein, we know the IPTG could not induce LacI promoter during the stationary phase.</td> |
| - | + | </tr></table> | |
| - | + | ||
| - | + | <table width="99%" cellpadding="0" cellspacing="10" style="padding: 10px; background-color: #FFFF99"> | |
| - | b. The minimum concentration of IPTG required to induce the lac promoter is very low and has no direct influence on the final result | + | <tr> |
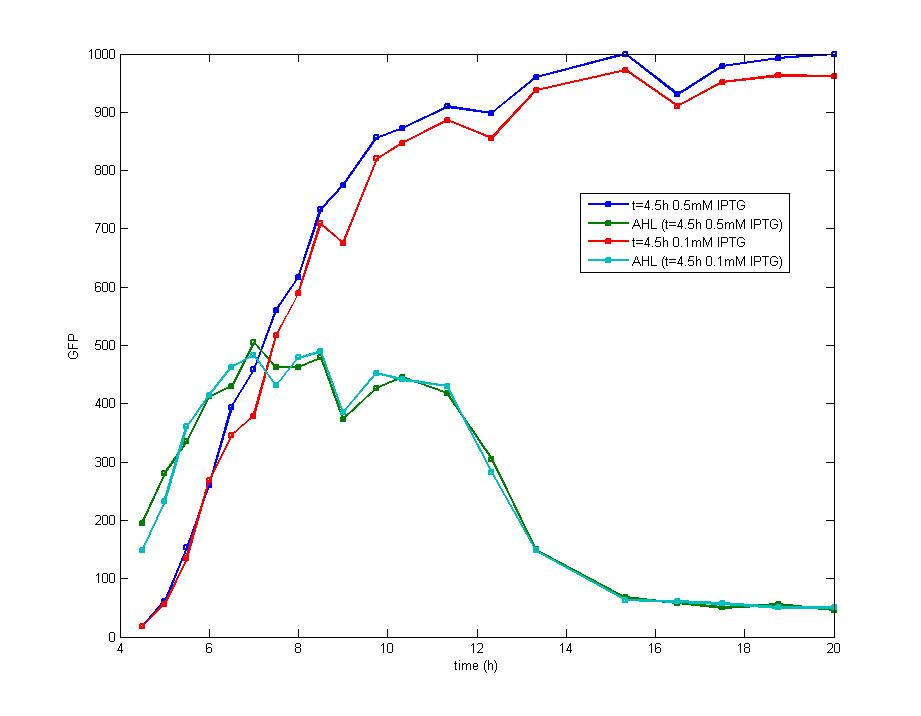
| + | <td>Figure 4. The concentration of extracellular AHL is examined after the induction of diluted IPTG and it seems there is no difference between the results of 0.5mM IPTG and 0.1mM IPTG. The concentration of AHL decreases remarkably during the stationary phase which could be attributed to the fast degradation rate and the ceasing of IPTG induction.</td> | ||
| + | <td>[[Image:tjuexpff204.jpg|300px]]</td> | ||
| + | </tr></table> | ||
| + | |||
| + | <table width="99%" cellpadding="0" cellspacing="10" style="padding: 10px; background-color: #FFFF99"> | ||
| + | <tr> | ||
| + | <td>[[Image:tjuexpff205.jpg|300px]]</td> | ||
| + | <td>Figure 5. The addition of IPTG with a concentration of 1 mM and 0.1 mM respectively at the inoculation makes no diversity on the induction behavior ,but the fluorescence intensity varies at the higher level.</td> | ||
| + | </tr></table> | ||
| + | |||
| + | According to the above analysis, we conclude:<br> | ||
| + | a. The early exponential phase is most suitable for the addition of IPTG which could repress the expression of luxR effectively and provide us more time to get detail information before cells enter the stationary phase. The optimal bacteria density for the addition of IPTG is the range of 0.2(OD<sub>600</sub>) to 0.3(OD<sub>600</sub>).<br> | ||
| + | b. The minimum concentration of IPTG required to induce the lac promoter is very low and has no direct influence on the final result. Besides, the induction of IPTG is so focused on the beginning that it could be treated as an instant signal, it is unnecessary to add more IPTG after the first addition. The optimal concentration of IPTG for induction is 0.5mM. | ||
<br><br> | <br><br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| + | [[Image:tjuexpff206.jpg|500px]]<br> | ||
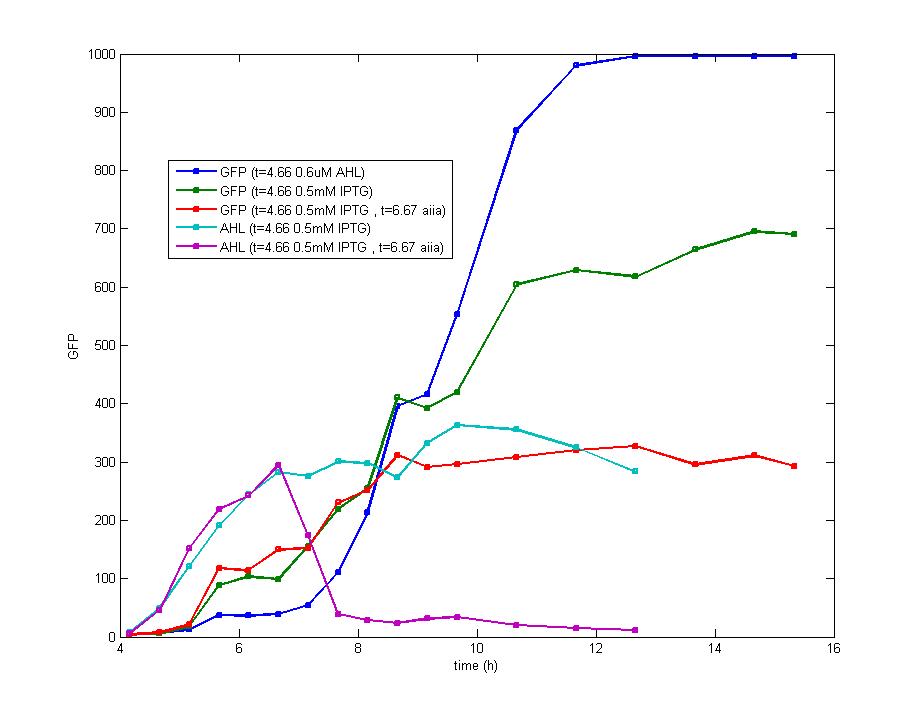
| + | Figure 6. AHL exists in the culture during the whole exponential phase as the blue line displays. However, the green curve implies that the expression of fluorescence protein has been ceased 6 hours after the induction. Therefore, cI promoter has been inhibited to express luxR protein and original LuxR protein has been combined as well as degraded to a low level that fails to initialize the expression of fluorescence protein, which matches the model of Flip-Flop perfectly. | ||
| + | On the other hand, the fluorescence intensity of cells with the addition of AHL tends to be higher than the addition of IPTG, proving the addition of IPTG inhibits the activity of cI promoter which reduces the production of LuxR. | ||
| + | The fluorescence intensity maintains stable rather than declines even after 16 hours since induction which could be ascribed to the long half life of fluorescence protein which is hardly degraded during the growth of bacteria. As our experiment proved, the fluorescence intensity still reaches 80% of the peak value after 40- hour culturation, which is the bottleneck for our biological Flip-Flop. The addition of Aiia protein (a protein digesting AHL) 2 hours after the addition of IPTG nearly consumes all the extracellular AHL, but the fluorescence intensity hardly changes. Therefore, in our experiment, every part of the trigger device functions as well as we expected, the only problem is to abbreviate the degradation period of fluorescence protein. Once this problem could be solved, better characters of Flip-Flop are expected to be observed. | ||
|} | |} | ||
| + | [http://www.example.com link title] | ||
Latest revision as of 02:45, 27 October 2007
[http://www.example.com link title]