Virginia Tech/rxn eqns
From 2007.igem.org
< Virginia Tech(Difference between revisions)
BlairLyons (Talk | contribs) m |
BlairLyons (Talk | contribs) m |
||
| Line 42: | Line 42: | ||
<img src="https://static.igem.org/mediawiki/2007/d/d5/Button_jc.JPG"/></a></html>[[Image:Dot1.JPG]] | <img src="https://static.igem.org/mediawiki/2007/d/d5/Button_jc.JPG"/></a></html>[[Image:Dot1.JPG]] | ||
| - | <!--sixth cell of second table: | + | <!--sixth cell of second table: Contributions and Contact--> |
| style="padding: 0px; width=50px; background-color: #FFFFFF;" | | | style="padding: 0px; width=50px; background-color: #FFFFFF;" | | ||
| - | <html><a href="https://2007.igem.org/Virginia_Tech/ | + | <html><a href="https://2007.igem.org/Virginia_Tech/Contributions"> |
| - | <img src="https://static.igem.org/mediawiki/2007/ | + | <img src="https://static.igem.org/mediawiki/2007/6/69/Button_contribute.JPG"/></a></html><html><a href="mailto:igem@vt.edu"></a></html> |
|}<html></center></html> | |}<html></center></html> | ||
Latest revision as of 03:23, 27 October 2007
|
|
|
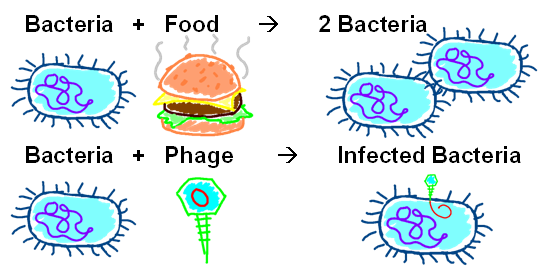
Modeling with reaction equations allows for flexibility in our modeling environment.Reaction equations are like chemical equations, except their species are not always chemicals. Reaction equations can be modeled differentially or stochastically, making them a good fit for our model. To the right are examples of two reaction equations that are used in our model. We can also add other species like lytic bacteria, lysogenic bacteria, and space. Reaction equations can be controlled using rates, constants, initial concentrations, and intermediate steps. |