Valencia/bacterial hysteresis cycle --a first approach
From 2007.igem.org
this is a first approach to some thoughs Emilio and I put on the table some time ago. it is not a complicated matter (at least in its biological part) and if we are able to do its characterization, we would deserve something better than an IgNobel.
if anyone feels like as if contributing to the idea, feel free. here we go:
the idea is to work with promoters that drive different quantity of a given reporter (let's say GFP). the hysteresis cycle seems to be a kind of system's behaviour that is very useful to engineers (don't ask me why, yet). looking at a typical histeresis cycle, I thought that a time-based graph would work better and would make more biological sense.
 this a typical hysteresis cycle from a google-ing
this a typical hysteresis cycle from a google-ing
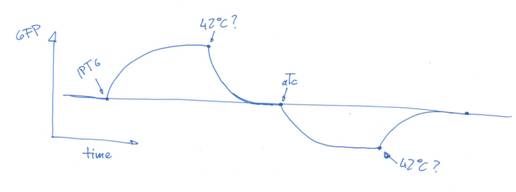 and this is my time-based interpretation
and this is my time-based interpretation
so, this way, we have a reporter that acts accordingly to three external inputs. for example: IPTG, temperature and aTc. the 'cycle' would be:
-at time 0, it has a given (very low) activity (so low, we may say that it's none)
-at time 1, we put some concentration of IPTG, and the system increases its fluorescence (if we are to use GFP)
-then, we use a different input, temperature, and the systems goes to the basal state (problem: the system would need a constant concentration of IPTG to be stable, but those are technical problems)
-now is when the fun starts: with the third input, we reach a lower reporter concentration
-and, our temperature shift makes the system go back to the initial state.
now, one of the most beatiful things one can found in this so-called synthetic biology is to step trough the mirror that divides the pencil and the biology as well as the one between the biology and the computer. for example: how are we going to get this behaviour in a bacteria??
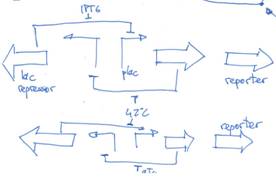
one possible response: using two toggle switches... (and excuse me for the poor images)
according to the [http://www.nature.com/nature/journal/v403/n6767/full/403339a0.html paper] (else e-mail me), two repressors and two inducers could regulate the system. my idea is 'just' couple two toggle switches with a common repressor (i.e., the 42ºC induction). Whenever you put the bacteria at 42ºC you get the 'initial state'. IPTG makes the reporter being produced in higher yields and aTc makes the reporter concetration go low.
if you're into molecular biology, you may think that the average life time of the proteins may blur the whole system up. that's why we'll have to use tagged repressors and GFP (or whichever reporter we use). see it [http://en.wikipedia.org/wiki/Repressilator here].
problems:
-having constant concentrations of inducers
-time course may be important
-the promoters should be found and be available
-technical problems may be found on the way (of course)
-the GFP data normalization can be critical --but, hey, if this would be easy, it wouldn't be science!!
--arnau
Take two:
In order to this be a real hysteresis cycle, the 42ºC input not be common to both ‘toggle switches’. This is easy to solve: building two different toggle switches with different inputs.
I still like the idea of a rough 'proteostate' that can be put to zero with a given input, but my physicist roommate reminds me that this kind of device is not a hysteresis cycle.
So, having two toggle switches coupled we have a project and decoupling them, we have another project... --arnau