Paris/July 20
From 2007.igem.org
< Paris(Difference between revisions)
Nicolas C. (Talk | contribs) |
|||
| (12 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | [[Paris/July 19|yesterday]] -- [[Paris/July 21|tomorrow]] <br> | ||
==PCRs== | ==PCRs== | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
{{Paris_PCR_0| Title = Lox71-KmR-Lox66 | {{Paris_PCR_0| Title = Lox71-KmR-Lox66 | ||
| - | |Annealing= | + | |Annealing= 55 |
| - | |Elongation= | + | |Elongation= 3m00' |
| - | |Cycles= | + | |Cycles= 35 |
|Buffer= 5x 10µL | |Buffer= 5x 10µL | ||
|MgCl2= 10µM 0µL | |MgCl2= 10µM 0µL | ||
| Line 19: | Line 13: | ||
|n_oligoR= 11 Lox66-KmR-R | |n_oligoR= 11 Lox66-KmR-R | ||
|v_oligoR= 2.5µL | |v_oligoR= 2.5µL | ||
| - | |water= | + | |water= 34µl |
|pol= Phusion 0.5µL | |pol= Phusion 0.5µL | ||
| - | |DNA= MP3_pSB1AK3-BBa_B0015 | + | |DNA= MP3_pSB1AK3-BBa_B0015 0.5µl |
| - | |Size= | + | |Size= 1084 |
| - | |Success= | + | |Success= ? |
| + | |Image= | ||
| + | |Band= | ||
| + | |}} | ||
| + | |||
| + | {{Paris_PCR_0| Title = Lox71-FtsA-FtsZ | ||
| + | |Annealing= 55 | ||
| + | |Elongation= 3m00' | ||
| + | |Cycles= 35 | ||
| + | |Buffer= 5x 10µL | ||
| + | |MgCl2= 10µM 0µL | ||
| + | |dNTP= 10µM 1µL | ||
| + | |n_oligoF= 3 Lox71-FtsA-F | ||
| + | |v_oligoF= 2.5µL | ||
| + | |n_oligoR= 2 FtsZ-R | ||
| + | |v_oligoR= 2.5µL | ||
| + | |water= 34µl | ||
| + | |pol= Phusion 0.5µL | ||
| + | |DNA= toothpick in MG1655 glycerol | ||
| + | |Size= 2588 | ||
| + | |Success= ? The PCR seemed to have worked but the purification failed | ||
|Image= | |Image= | ||
|Band= | |Band= | ||
| Line 30: | Line 44: | ||
==Glycerol stock== | ==Glycerol stock== | ||
| - | * BBa_pJ23107 clone 3&4 | + | * BBa_pJ23107 clone 3&4 (S14) |
| - | * BBa_I0500 clone 1&2 | + | * BBa_I0500 clone 1&2 (S13) |
==Minipreps on Biobricks== | ==Minipreps on Biobricks== | ||
| Line 37: | Line 51: | ||
* BBa_pJ23107 clone 3&4 | * BBa_pJ23107 clone 3&4 | ||
* BBa_I0500 clone 1&2 | * BBa_I0500 clone 1&2 | ||
| + | |||
| + | == E.Coli pKS::DGAT == | ||
| + | |||
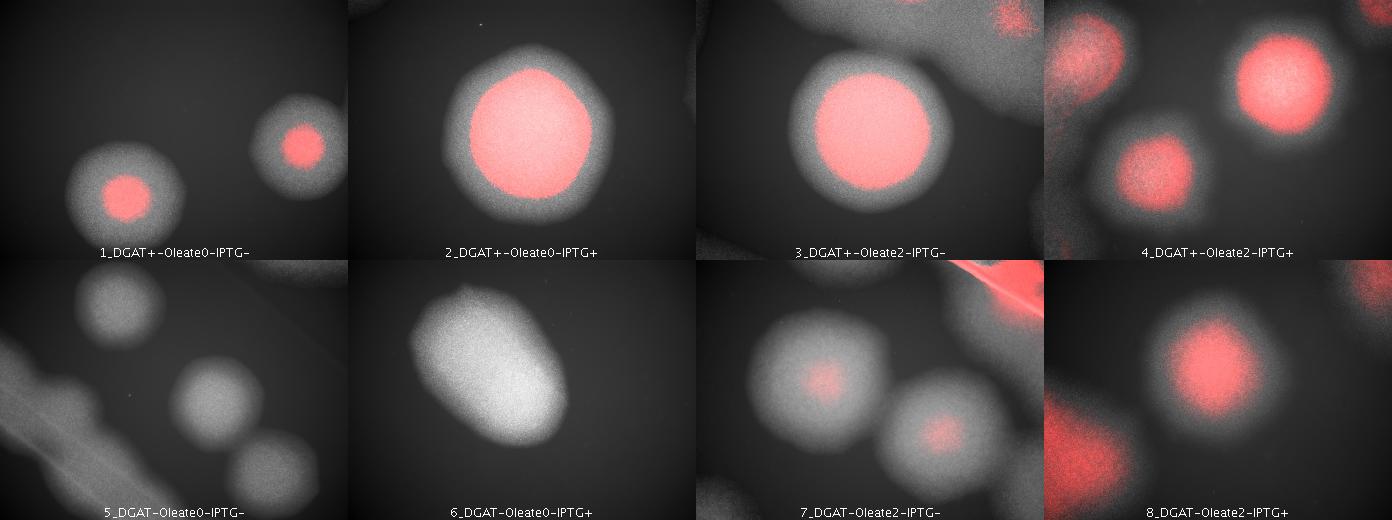
| + | We look under microscopy 36 hours after incubation of E.coli transformed by pKS::DGAT and the control E.coli transformed by part B0015 on different LB medium (See [[Paris/July_18#E.coli_pKS::DGAT|July 18]]). | ||
| + | |||
| + | * Observations: | ||
| + | We can observe colonies (X10). | ||
| + | [[Image: coli_dgat_07202007.jpg|center|900px]] | ||
| + | |||
| + | * Interpretation | ||
| + | |||
| + | We can observe lipid inclusions within colonies with DGAT. Further investigations need single cell visualisation. | ||
Latest revision as of 17:49, 7 October 2007
Contents |
PCRs
| PCR : Lox71-KmR-Lox66 | ||||||
|---|---|---|---|---|---|---|
| PCR Settings | Buffer (5x) | 5x 10µL | Expected size | |||
| Annealing (°C) | MgCl2 10µM | 10µM 0µL | 1084 | |||
| 55 | dNTP 10µM | 10µM 1µL | Success | |||
| Time Elongation | Oligo F 10µM | 10 Lox71-KmR-F | 2.5µL | ? | ||
| 3m00' | Oligo R 10µM | 11 Lox66-KmR-R | 2.5µL | Image (click to enlarge) | ||
| Number cycles | Water | 34µl | [[Image:|30px]] | |||
| 35 | Polymerase | Phusion 0.5µL | Band (0=ladder) | |||
| DNA | MP3_pSB1AK3-BBa_B0015 0.5µl | |||||
| PCR : Lox71-FtsA-FtsZ | ||||||
|---|---|---|---|---|---|---|
| PCR Settings | Buffer (5x) | 5x 10µL | Expected size | |||
| Annealing (°C) | MgCl2 10µM | 10µM 0µL | 2588 | |||
| 55 | dNTP 10µM | 10µM 1µL | Success | |||
| Time Elongation | Oligo F 10µM | 3 Lox71-FtsA-F | 2.5µL | ? The PCR seemed to have worked but the purification failed | ||
| 3m00' | Oligo R 10µM | 2 FtsZ-R | 2.5µL | Image (click to enlarge) | ||
| Number cycles | Water | 34µl | [[Image:|30px]] | |||
| 35 | Polymerase | Phusion 0.5µL | Band (0=ladder) | |||
| DNA | toothpick in MG1655 glycerol | |||||
Glycerol stock
- BBa_pJ23107 clone 3&4 (S14)
- BBa_I0500 clone 1&2 (S13)
Minipreps on Biobricks
- BBa_pJ23107 clone 3&4
- BBa_I0500 clone 1&2
E.Coli pKS::DGAT
We look under microscopy 36 hours after incubation of E.coli transformed by pKS::DGAT and the control E.coli transformed by part B0015 on different LB medium (See July 18).
- Observations:
We can observe colonies (X10).
- Interpretation
We can observe lipid inclusions within colonies with DGAT. Further investigations need single cell visualisation.