Glasgow/Goals/FuelCells
From 2007.igem.org
| Line 13: | Line 13: | ||
<font face=georgia color=#0000FF size=4>Our Own Microbial Fuel Cells</font><br> | <font face=georgia color=#0000FF size=4>Our Own Microbial Fuel Cells</font><br> | ||
<br> | <br> | ||
| - | |[[Image:IMG | + | |[[Image:IMG 4468.JPG|250px]] |
{| cellspacing="6px" cellpadding="16" border="0" width="100%" | {| cellspacing="6px" cellpadding="16" border="0" width="100%" | ||
|- | |- | ||
Revision as of 14:35, 9 October 2007
| https://static.igem.org/mediawiki/2007/thumb/c/cc/Uog.jpg/50px-Uog.jpg | Back To Glasgow's Main Page | Back To Glasgow's Project Page |
|---|
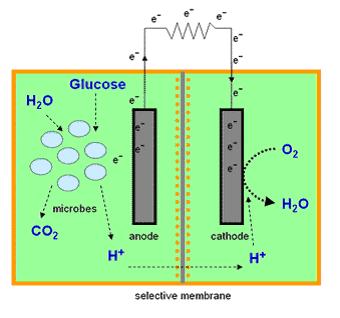
Microbial Fuel Cells
Most microbial cells are electrochemically inactive. The electron transfer from microbial cells to the electrode is facilitated by mediators such as thionine, methyl viologen (methyl blue), neutral red etc, and of the mediators available are expensive and toxic. Microbial fuel cells produce power by use of a microbial cell-permeable chemical mediator, which in the oxidised form intercepts a proportion of NADH (nicotinamide adenine dinucleotide) within the microbial cell and oxidises it to NAD+. The now reduced form of mediator is also cell-permeable and diffuses away from the microbial cell to the anode where, the reduced redox mediator is then electro-catalytically re-oxidised. In addition, cell metabolism produces protons in the anodic chamber, which may migrate through a proton selective membrane to the cathodic chamber. In the latter, they are consumed by ferricyanide (Fe3-(CN)6) and incoming electrons (via the external circuit) reducing it to ferrocyanide (Fe4-(CN)6 ). The oxidised mediator is then free to repeat the cycle. This cycling continually drains off metabolic reducing power from the microbial cells to give electrical power at the electrodes.
Our Own Microbial Fuel Cells
|

| 
| 
|
| 
| 
|