Glasgow/Wetlab/Results
From 2007.igem.org
EmmaTravis (Talk | contribs) (→<font face=georgia color=#0000FF size=4>DntR and Dinitrotoluenes</font><br>) |
EmmaTravis (Talk | contribs) (→<font face=georgia color=#0000FF size=4>XylR and BETX Chemicals</font><br>) |
||
| (11 intermediate revisions not shown) | |||
| Line 12: | Line 12: | ||
== <font face=georgia color=#0000FF size=4>XylR and BETX Chemicals</font><br> == | == <font face=georgia color=#0000FF size=4>XylR and BETX Chemicals</font><br> == | ||
| - | |||
| - | |||
| - | + | The XylR transcriptional regulator system was initially tested for the induction of the reporter gene lacZ driven by the Pu promoter. Initial studies were not performed in a BioBrick system as they were occurring in parallel with the creation of novel BioBricks. Instead, a construct based on the pQF52 reporter plasmid containing a Pu:lacZ fusion reporter gene was utilised. The XylR regulatory gene was cloned in the opposite orientation. The plasmid was termed pQF52XylR | |
| - | + | ||
| - | + | ||
| - | + | ||
<br> | <br> | ||
''E. coli'' carrying pQF52XylR were grown in LB culture overnight before addition of a range of aromatic environmental pollutants were added. Production of lacZ was quantified by the Miller Assay ([[Glasgow/Wetlab/Protocols#Protocol 10: Miller Assay|Protocol 10]]).<br> | ''E. coli'' carrying pQF52XylR were grown in LB culture overnight before addition of a range of aromatic environmental pollutants were added. Production of lacZ was quantified by the Miller Assay ([[Glasgow/Wetlab/Protocols#Protocol 10: Miller Assay|Protocol 10]]).<br> | ||
| Line 26: | Line 21: | ||
<br> | <br> | ||
The XylR system was considered suitable for further development and so [http://partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2007&group=Glasgow BioBricks were constructed] to facilitate production of a biosensor that generates pyocyanin. In order to test alternative reporter systems, [http://partsregistry.org/Part:BBa_ a sensor system where XylR transcriptionally regulates the production of the Renilla luciferase (BBa_I723023)] has been successfully created using the RBS [http://partsregistry.org/Part:BBa_J61101 BBa_J61101] and the luciferase CDS [http://partsregistry.org/Part:BBa_J52008 BBa_J52008].<br> | The XylR system was considered suitable for further development and so [http://partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2007&group=Glasgow BioBricks were constructed] to facilitate production of a biosensor that generates pyocyanin. In order to test alternative reporter systems, [http://partsregistry.org/Part:BBa_ a sensor system where XylR transcriptionally regulates the production of the Renilla luciferase (BBa_I723023)] has been successfully created using the RBS [http://partsregistry.org/Part:BBa_J61101 BBa_J61101] and the luciferase CDS [http://partsregistry.org/Part:BBa_J52008 BBa_J52008].<br> | ||
| + | |||
| + | Luminescence increased at higher concentrations of toluene, indicative of increased luciferase expression under the control of Pu. Experiments are continuing to provide quantitative data using a luminometer and using different environmental pollutants. | ||
| + | <br> | ||
| + | {| align=center border=0 | ||
| + | |- | ||
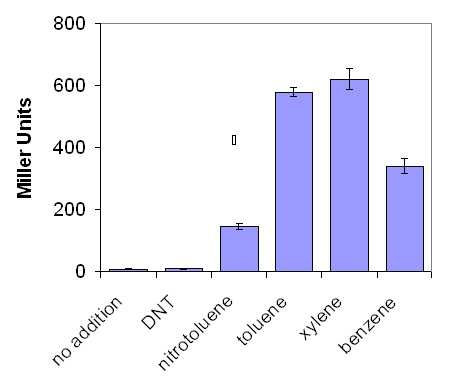
| + | |[[Image:Graph1b.PNG|frame|'''Figure 1a:''' The effect of a variety of environmental pollutants on lacZ production by ''E. coli'' containing pQF52 XylR, when grown on LB media, as measured by the Miller Assay.]] | ||
| + | |[[Image:Glowing Bugs.jpg|frame|'''Figure 1b:''' Luminescence increased as toluene concentrations increased. The top photograph shows the luminescence when an image was taken in the dark. The lower photograph shows the same tubes when in the light. The contents of the tubes are indicated.]] | ||
| + | |} | ||
| + | |||
<br> | <br> | ||
Latest revision as of 17:13, 26 October 2007
 | Back To Glasgow's Main Page | Back To Glasgow's Wetlab Log | Back To Glasgow's Project Page |
|---|
Contents[hide] |
Results
Pyocyanin
The genes encoding the two final enzymes involved in the pyocyanin biosynthesis pathway, PhzM and PhzS, were cloned and successfully made into BioBricks. They have been subcloned to include terminators after the genes. The phenazine biosynthesis operon responsible for the production of the pyocyanin precursor phenazine-1-carboxylic acid (PCA) was over 7kb long and proved troublesome to clone. An alternative strategy involved cloning this operon in two parts. PhzE, PhzF and PhzG were cloned into BioBrick format and are currently undergoing site directed mutagenesis. Pseudomonas fluorescens 2-71 was obtained as an alternative source of PCA.
XylR and BETX Chemicals
The XylR transcriptional regulator system was initially tested for the induction of the reporter gene lacZ driven by the Pu promoter. Initial studies were not performed in a BioBrick system as they were occurring in parallel with the creation of novel BioBricks. Instead, a construct based on the pQF52 reporter plasmid containing a Pu:lacZ fusion reporter gene was utilised. The XylR regulatory gene was cloned in the opposite orientation. The plasmid was termed pQF52XylR
E. coli carrying pQF52XylR were grown in LB culture overnight before addition of a range of aromatic environmental pollutants were added. Production of lacZ was quantified by the Miller Assay (Protocol 10).
LacZ production was greatest in response to xylene but also reached comparable levels when the bacteria were challenged with benzene and toluene (figure 1). This would be of benefit to an environmental system where the aim is purely to detect the incidence of pollution, reducing the materials and costs associated with sensing as many different chemicals as possible. XylR was not deemed sensitive enough to nitrotoluene and dinitrotoluene (DNT) to do any further investigation with those pollutants.
The XylR system was considered suitable for further development and so [http://partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2007&group=Glasgow BioBricks were constructed] to facilitate production of a biosensor that generates pyocyanin. In order to test alternative reporter systems, [http://partsregistry.org/Part:BBa_ a sensor system where XylR transcriptionally regulates the production of the Renilla luciferase (BBa_I723023)] has been successfully created using the RBS [http://partsregistry.org/Part:BBa_J61101 BBa_J61101] and the luciferase CDS [http://partsregistry.org/Part:BBa_J52008 BBa_J52008].
Luminescence increased at higher concentrations of toluene, indicative of increased luciferase expression under the control of Pu. Experiments are continuing to provide quantitative data using a luminometer and using different environmental pollutants.
DntR and Dinitrotoluenes
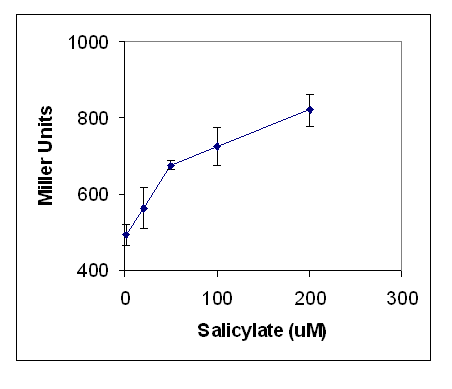
The DntR transcriptional regulator system was tested for the induction of the reporter gene lacZ, linked to the DntA promoter. Initial studies were not performed in a BioBrick system as they were occurring in parallel with the creation of novel BioBricks. Instead, a construct based on the pQF52 reporter plasmid containing a DntA:lacZ fusion reporter gene was used (DntA is activated by salicylate-bound DntR). The DntR regulatory gene was cloned in the opposite orientation. The plasmid was termed pQF52DntR.
E. coli carrying pQF52DntR were grown in LB culture overnight before addition of a range of concentrations of salicylate. Production of lacZ was quantified by the Miller Assay (Protocol 10).
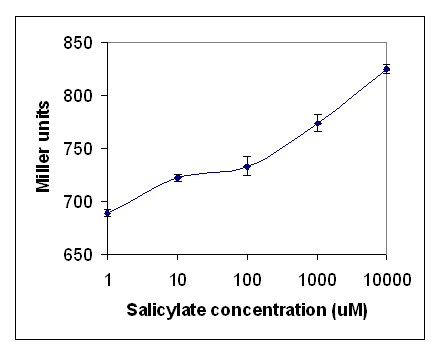
LacZ production increased significantly as salicylate concentration increased (Figure 2). A background production of lacZ was observed, therefore subsequent experiments were designed to limit any chemical species that might agonise the receptor. Minimal medium was used to limit the amounts of tryptophan - a potential mimic of salicylate. The range of salicylate concentrations was extended to determine the linearity of the response. The results are shown in Figure 3. There was no reduction in background activity. As salicylate concentration increased, the response increased, showing that the assay is sensitive from 10um to at least 10mM.
The DntR system was considered suitable for further development and so [http://partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2007&group=Glasgow BioBricks were constructed] to facilitate production of a biosensor that generates pyocyanin.
DmpR and Phenolic Compounds
The DmpR transcriptional regulator system was tested for the induction of the reporter gene lacZ driven by the Dmp operon promoter. Initial studies were not performed in a BioBrick system as they were occurring in parallelwith the creation of novel BioBricks. Instead, we used a construct obtained by personal communication with A. Wise containing a Dmp-target:lacZ fusion reporter gene chromosomally inserted at the trp locus. The trainscriptional regulator DmpR was carried on a plasmid – p322-dmpR (RI) - which was a derivative of pBR322.
E. coli containing the chromosomal insert and the plasmid p322-dmpR (RI) were grown on a variety of agar media. We tested rich media (Luria-Bertani - LB), minimal media and spent LB media. The colonies were blotted onto a sheet of hybrid N nylon membrane. The membrane was placed in a petri dish on top of thin layer of agar containing X-gal before adding phenol, cresols, dinitrophenols, dimethylphenols and dichlorophenols and incubated for 2 hrs at 37 C. The membranes were then assessed for the development of blue colouration.
There was high background production of lacZ in all of the media tried, but the spent LB had the lowest. This was thought to be due to the presence of the amino acid tryptophan in the media. This amino acid is essential for the growth of E. coli. Its chemical structure is similar to phenol and could therefore be triggering expression of lacZ.
It was decided that due to the background induction of lacZ by tryptophan, the transcriptional regulator DmpR would not be studied further. The production of the relevant biobricks was therefore halted.