Tokyo/Works/Assay2
From 2007.igem.org
| (80 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
| - | <br>[[Tokyo/Works|Works top]] | + | <br>[[Tokyo/Works|Works top]] 0.[[Tokyo/Works/Hybrid promoter|Hybrid promoter]] 1.[[Tokyo/Works/Formulation |Formulation]] 2.[[Tokyo/Works/Assay |Assay1]] 3.[[Tokyo/Works/Simulation |Simulation]] 4.[[Tokyo/Works/Assay2 |Assay2]] 5.[[Tokyo/Works/Future works |Future works]] |
| + | <br><br> | ||
| + | [[Tokyo/Activation check by cell-produced AHL |Activation check by cell-produced AHL ]] [[Tokyo/Expression level check|Expression level check on ''promoters + plasmid sets'' of A and B sides]] | ||
| + | ==Objective== | ||
| + | <!--<br>Fomulationで建てた式に必要なパラメータをAssay1により実験的に求めた。それを導入した結果を解析したところ、以下の実験の必要性が浮かび上がった。--> | ||
| - | + | Parameters for the equations in [[Tokyo/Works/Formulation |Formulation]] have been experimentally determined in [[Tokyo/Works/Assay |Assay1]]. On the basis of the results of [[Tokyo/Works/Simulation |the simulations]], the following experiments were carried out to identify the nature of the current circuit construction. | |
| - | == | + | == [[Tokyo/Activation check by cell-produced AHL |Activation check by cell-produced AHL ]] == |
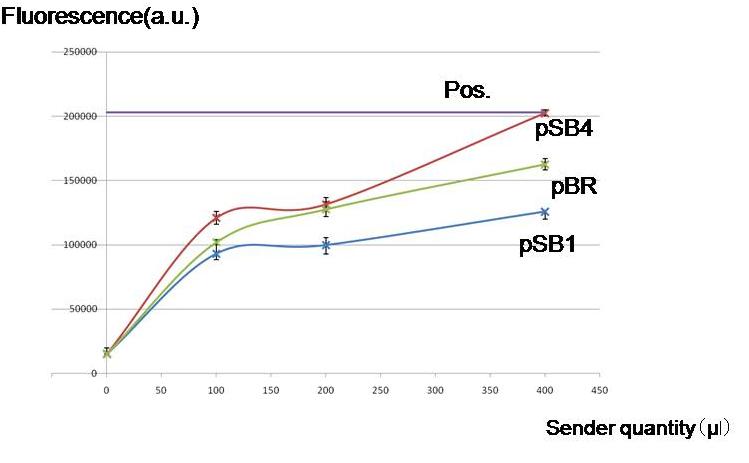
| + | [[Image:Endogenous.jpg|thumb|300px| '''Fig.1''' By culturing with AHL producing cells, whether the lux lac hybrid promoter was activated without externally adding AHL. Regardless of the copy numbers of the luxI encoding plasmids, the hybrid promoter was activated.]] | ||
| + | '''Objective ''' | ||
| + | |||
| + | To check whether worker E. coli (Sender) can produce enough amount of AHL for our model to work by using different copy numbers of plasmids | ||
| + | |||
| + | |||
| + | '''Result & Conclusion''' | ||
| + | |||
| + | Not only a high-copy-number-plasmid pSB1 derivative, but also low copy number plasmid pSB4- and pBR derivatives produced enough AHL to activate the LacI hybrid promoter in other cells. Especially, the pBR derivative planned to use in the current construction, remarkably produced AHL in the present experiments. | ||
| + | <br> | ||
| + | |||
| + | <br> | ||
| + | |||
| + | ==== ==> see more [[Tokyo/Activation check by cell-produced AHL|details]]==== | ||
| + | |||
| + | <br> | ||
| + | |||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | |||
| + | ==[[Tokyo/Expression level check|Expression level check on ''promoters + plasmid sets'' of A and B sides]]== | ||
| + | |||
| + | '''Objective ''' | ||
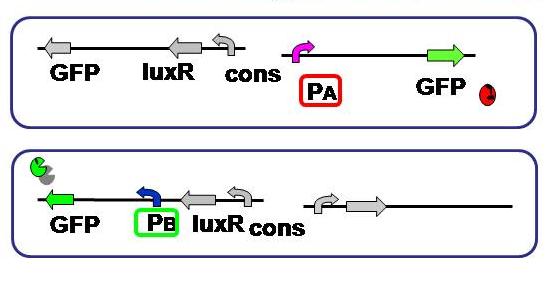
| + | [[Image:promoter hikaku.JPG|thumb|300px| '''Fig.2''' Expression levels of the both sides were compared. As to the A side, RFP was substituted with GFP.]] | ||
| + | |||
| + | <br>Aimulations indicated that not only relative AHL production efficiency, but production ratio of a repressor to the other is important for balanced differentiation. To test and compare the gene expression level of each side, A and B of the current genetic circuit construction. | ||
| + | Since the cell type - A or B - is detected based on the fluorescence, its activity should be measured and standardized beforehand. | ||
| + | Here we used the same fluorescent protein GFP on the both promoter + plasmid sets actually used in our model, where A side consists of Lambda cI-regulated promoter, and B side the lux lac hybrid promoter. | ||
| + | <br><br> | ||
| + | '''Result & Conclusion''' | ||
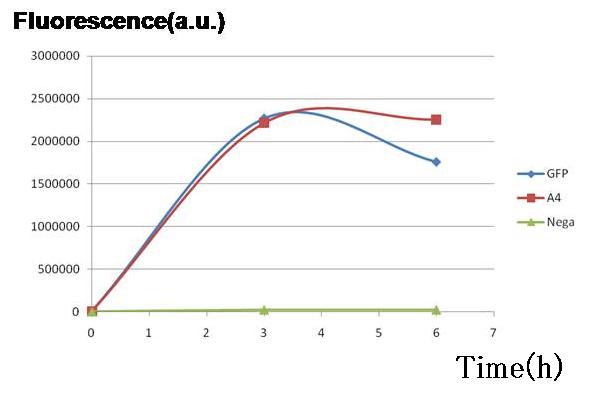
| + | <br>Two plasmid sets, A4 ΔP/pc1-GFP(upper side of Fig.2, off/on respectively) and A4 hybrid-promoter(+AHL to activate)/pBR322tetR (lower side of Fig.2, on/off respectively), showed almost the same fluorescence of GFP. This result indicates that expression levels of both sets were almost the same, though the latter was a bit smaller. In the next construction, RBS and/ or promoter modifications are required to adjust the expression balance of the repressors. [[Image:pC1GFP.jpg|thumb|300px| '''Fig.3''' Genes downstream of the promoters on the both sides were expressed to almost the same degrees in terms of GFP fluorescence.]] | ||
| + | <br><br> | ||
| + | |||
| + | |||
| + | |||
| + | ==== ==> see more [[Tokyo/Expression level check|details]]==== | ||
Latest revision as of 04:29, 27 October 2007
Works top 0.Hybrid promoter 1.Formulation 2.Assay1 3.Simulation 4.Assay2 5.Future works
Activation check by cell-produced AHL Expression level check on promoters + plasmid sets of A and B sides
Objective
Parameters for the equations in Formulation have been experimentally determined in Assay1. On the basis of the results of the simulations, the following experiments were carried out to identify the nature of the current circuit construction.
Activation check by cell-produced AHL
Objective
To check whether worker E. coli (Sender) can produce enough amount of AHL for our model to work by using different copy numbers of plasmids
Result & Conclusion
Not only a high-copy-number-plasmid pSB1 derivative, but also low copy number plasmid pSB4- and pBR derivatives produced enough AHL to activate the LacI hybrid promoter in other cells. Especially, the pBR derivative planned to use in the current construction, remarkably produced AHL in the present experiments.
==> see more details
Expression level check on promoters + plasmid sets of A and B sides
Objective
Aimulations indicated that not only relative AHL production efficiency, but production ratio of a repressor to the other is important for balanced differentiation. To test and compare the gene expression level of each side, A and B of the current genetic circuit construction.
Since the cell type - A or B - is detected based on the fluorescence, its activity should be measured and standardized beforehand.
Here we used the same fluorescent protein GFP on the both promoter + plasmid sets actually used in our model, where A side consists of Lambda cI-regulated promoter, and B side the lux lac hybrid promoter.
Result & Conclusion
Two plasmid sets, A4 ΔP/pc1-GFP(upper side of Fig.2, off/on respectively) and A4 hybrid-promoter(+AHL to activate)/pBR322tetR (lower side of Fig.2, on/off respectively), showed almost the same fluorescence of GFP. This result indicates that expression levels of both sets were almost the same, though the latter was a bit smaller. In the next construction, RBS and/ or promoter modifications are required to adjust the expression balance of the repressors.