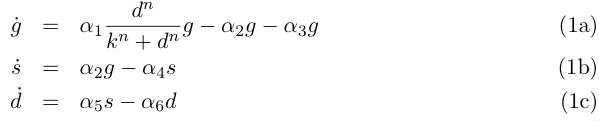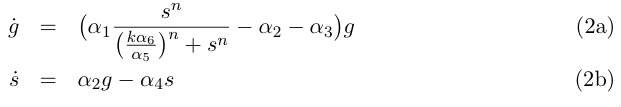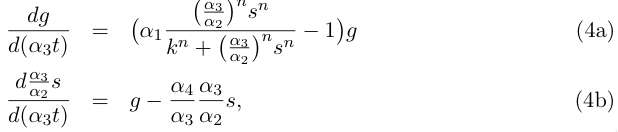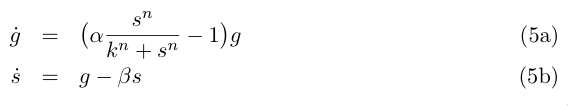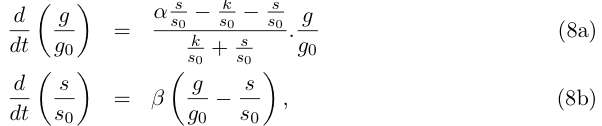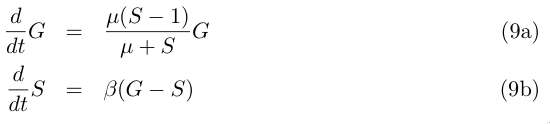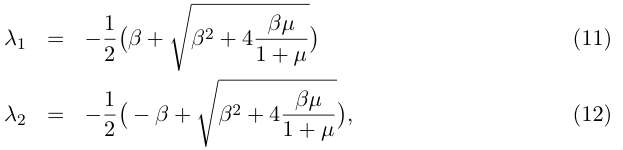Paris/Continuous model
From 2007.igem.org
(→Conclusion) |
|||
| (8 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Template:Paris_menu_modeling}} | {{Template:Paris_menu_modeling}} | ||
| + | <br> | ||
=Well mixed population model = | =Well mixed population model = | ||
We present here a theoretical approach based on population dynamics. We consider here | We present here a theoretical approach based on population dynamics. We consider here | ||
| - | the case of a well mixed, | + | the case of a well mixed, homogeneous, culture of the SMB organism, i.e. there is no space |
in this analysis and we follow only the variation of the different cell lines concentrations | in this analysis and we follow only the variation of the different cell lines concentrations | ||
in the culture volume. | in the culture volume. | ||
| Line 11: | Line 12: | ||
== Derivation of the model == | == Derivation of the model == | ||
| - | Let the variables ''g, s'' and ''d'' describe respectively the concentrations of germinal and | + | Let the variables ''g, s'' and ''d'' describe respectively the concentrations of germinal and somatic cells and the concentration of DAP, then we can write for our system: |
| - | + | ||
| - | [[Image:eq1.jpg]] | + | <br clear="all" /> |
| + | [[Image:eq1.jpg|right]] | ||
| + | <br clear="all" /> | ||
Equation (1a) describes the growth of the germinal cell population in presence of sufficient | Equation (1a) describes the growth of the germinal cell population in presence of sufficient | ||
| Line 22: | Line 25: | ||
The last line describes DAP production by the somatic cells, and includes a degradation | The last line describes DAP production by the somatic cells, and includes a degradation | ||
term. In absence of any quantitative details on assimilation of DAP by germinal cells and | term. In absence of any quantitative details on assimilation of DAP by germinal cells and | ||
| - | response to DAP levels, n and k are are to be considered as | + | response to DAP levels, n and k are are to be considered as arbitrary phenomenological |
parameters. We take however in the following n = 1 neglecting potential saturation related non-linearities for high DAP concentrations. The value of k corresponds to the DAP | parameters. We take however in the following n = 1 neglecting potential saturation related non-linearities for high DAP concentrations. The value of k corresponds to the DAP | ||
| - | concentration for half-maximal growth rate, and could set | + | concentration for half-maximal growth rate, and could set experimentally. |
We simplify the previous system by assuming that the evolution of d is rapid compared to | We simplify the previous system by assuming that the evolution of d is rapid compared to | ||
the cellular growth, so that at this time scale we can take d' = 0 and write d = s α5/ | the cellular growth, so that at this time scale we can take d' = 0 and write d = s α5/ | ||
| - | α6 . This gives the two-variable system : | + | α6 . This gives the two-variable system: |
| - | [[Image:eq2.jpg]] | + | <br clear="all" /> |
| + | [[Image:eq2.jpg|right]] | ||
| + | <br clear="all" /> | ||
| - | Redefinition of parameters k → kα6 /α5 and α3 → α2 + α3 leads to the simpler writing : | + | Redefinition of parameters k → kα6 /α5 and α3 → α2 + α3 leads to the simpler writing: |
| - | [[Image:eq3.jpg]] | + | <br clear="all" /> |
| + | [[Image:eq3.jpg|right]] | ||
| + | <br clear="all" /> | ||
| - | Let us do some rewriting : | + | Let us do some rewriting: |
| - | [[Image:eq4.jpg]] | + | <br clear="all" /> |
| + | [[Image:eq4.jpg|right]] | ||
| + | <br clear="all" /> | ||
| - | and by redefining the time and most of the parameters we get : | + | and by redefining the time and most of the parameters we get: |
| - | [[Image:eq5.jpg]] | + | <br clear="all" /> |
| + | [[Image:eq5.jpg|right]] | ||
| + | <br clear="all" /> | ||
| + | The fixed points are (g = 0, s = 0) and the solution of: | ||
| - | + | <br clear="all" /> | |
[[Image:eq6.jpg|right]] | [[Image:eq6.jpg|right]] | ||
<br clear="all" /> | <br clear="all" /> | ||
| + | |||
==Analysis of stability == | ==Analysis of stability == | ||
| + | |||
=== Fixed point at the origin === | === Fixed point at the origin === | ||
| - | Let us | + | |
| + | Let us linearize the system (5a) close to the origin. For small perturbations | ||
around (g = 0, s = 0) (5a) is equivalent to : | around (g = 0, s = 0) (5a) is equivalent to : | ||
<br clear="all" /> | <br clear="all" /> | ||
| Line 74: | Line 89: | ||
<br clear="all" /> | <br clear="all" /> | ||
| - | The fixed point is now (G0=1,S0=1). We | + | The fixed point is now (G0=1,S0=1). We linearize system (9a) around this point and |
look for a solution close to it. Take x := G−G0 and y := S−S0 , close to (G0,S0) we can | look for a solution close to it. Take x := G−G0 and y := S−S0 , close to (G0,S0) we can | ||
| - | write by keeping only the first order | + | write by keeping only the first order term of (9a) : |
<br clear="all" /> | <br clear="all" /> | ||
| Line 102: | Line 117: | ||
For a region around the origin and below some line passing through (G0, S0) any initial | For a region around the origin and below some line passing through (G0, S0) any initial | ||
| - | conditions converges to the origin. For higher values of the initial | + | conditions converges to the origin. For higher values of the initial conditions we always |
| - | expect exponential growth of the populations. | + | expect exponential growth of the populations. Indeed, if in the unstable case, we extrapolate |
| + | the asymptotic behaviour of our system, (2), we expect both population to grow, at some | ||
| + | point the Michaelis-Menten term saturates, and equation (2a) becomes g' = (α1-α2-α3)g. | ||
| + | The solution is a exponential with a positif exponent in this case. | ||
| + | |||
| + | |||
| + | It is interesting for comparaison with experimental measurements to evaluate the asymptotic behaviour of the ratio of the growth rate over population size g'/g. For this discussion | ||
| + | and without loss of generality in the treatment (implies only a redefinition of the parameter α2 ) | ||
| + | we neglect cell death, we will also keep n = 1 for the reason mentioned before. From the | ||
| + | preceeding paragraph we see that tha population of g cannot grow for α1 = α2 , that is | ||
| + | when the time for cell division (α1)^(-1) is equal to the generation time of somatic cells (α2)^(-1) . | ||
| + | Both cell types have the same growth rate, in our system it means that for each germinal | ||
| + | cell division there is one recombination of the CRE-LOX box and the generation of one | ||
| + | somatic cell. This means that we need less than 50% recombination probability in order | ||
| + | for our system to work. For recombination rates α2 (for fixed α1 ) close to this limit, the | ||
| + | growth rate over population size curve as a function α2 is a straight line with negative | ||
| + | slope g'/g = α1 − α2 , going to zero at 50% recombination probability. | ||
Latest revision as of 20:11, 26 October 2007
Contents |
Well mixed population model
We present here a theoretical approach based on population dynamics. We consider here the case of a well mixed, homogeneous, culture of the SMB organism, i.e. there is no space in this analysis and we follow only the variation of the different cell lines concentrations in the culture volume.
Derivation of the model
Let the variables g, s and d describe respectively the concentrations of germinal and somatic cells and the concentration of DAP, then we can write for our system:
Equation (1a) describes the growth of the germinal cell population in presence of sufficient DAP (interaction represented by the Michaelis-Menten function) ; the term proportional to α2 is the differentiation into somatic cells by recombination of the CRE/LOX box, and the last term proportional to α3 is the germinal cells’ death. Equation (1b) is the variation of the somatic cells’ population, with the term proportional to α4 for somatic cells’ death. The last line describes DAP production by the somatic cells, and includes a degradation term. In absence of any quantitative details on assimilation of DAP by germinal cells and response to DAP levels, n and k are are to be considered as arbitrary phenomenological parameters. We take however in the following n = 1 neglecting potential saturation related non-linearities for high DAP concentrations. The value of k corresponds to the DAP concentration for half-maximal growth rate, and could set experimentally. We simplify the previous system by assuming that the evolution of d is rapid compared to the cellular growth, so that at this time scale we can take d' = 0 and write d = s α5/ α6 . This gives the two-variable system:
Redefinition of parameters k → kα6 /α5 and α3 → α2 + α3 leads to the simpler writing:
Let us do some rewriting:
and by redefining the time and most of the parameters we get:
The fixed points are (g = 0, s = 0) and the solution of:
Analysis of stability
Fixed point at the origin
Let us linearize the system (5a) close to the origin. For small perturbations
around (g = 0, s = 0) (5a) is equivalent to :
We look for a solution of the form ![]() , with
, with ![]() an arbitrary vector. The values of λ are the eigenvalues of the matrix in (7) and can be obtain here straightforwardly by
the characteristic polynomial : λ1 = −b, λ2 = −1, with respectively the eigenvectors
an arbitrary vector. The values of λ are the eigenvalues of the matrix in (7) and can be obtain here straightforwardly by
the characteristic polynomial : λ1 = −b, λ2 = −1, with respectively the eigenvectors ![]() .
.
The two eigenvalues are always negative : the origin is therefore always an attractive fixed point. For too weak initial concentrations of the two cellular types, the system is always going to die out.
Second fixed point, out of the origin
Let (g0 := β s0 , s0 := k/(α−1) ) be the non trivial solution of (6a). We can simplify the (5a) by
dividing the two equations respectively by g0 et s0 and by taking as new variables G := g/g0
and S := s/s0:
and with μ := α−1 = α1/α2− 1 :
The fixed point is now (G0=1,S0=1). We linearize system (9a) around this point and look for a solution close to it. Take x := G−G0 and y := S−S0 , close to (G0,S0) we can write by keeping only the first order term of (9a) :
Looking for a solution of the form ![]() , we need to find the eigenvalues of the matrix in
(10). Solving the characteristic polynomial gives :
, we need to find the eigenvalues of the matrix in
(10). Solving the characteristic polynomial gives :
In order to determine the stability of the fixed point, let us examine the signs of the eigenvalues : λ1 is always negative, while λ2 is positive if μ/1+μ > 0. That is, by returning to the original parameters of (1) if α1 > α2 (more growth than recombination). In this case (which is expected) the fixed point (G0, S0) is unstable and the populations of the two cell lines diverge.
Conclusion
If we put together the results on the two fixed points we get the situation represented on the following diagram :
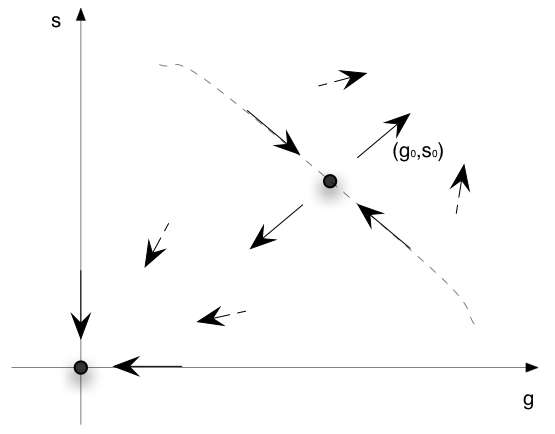
For a region around the origin and below some line passing through (G0, S0) any initial conditions converges to the origin. For higher values of the initial conditions we always expect exponential growth of the populations. Indeed, if in the unstable case, we extrapolate the asymptotic behaviour of our system, (2), we expect both population to grow, at some point the Michaelis-Menten term saturates, and equation (2a) becomes g' = (α1-α2-α3)g. The solution is a exponential with a positif exponent in this case.
It is interesting for comparaison with experimental measurements to evaluate the asymptotic behaviour of the ratio of the growth rate over population size g'/g. For this discussion
and without loss of generality in the treatment (implies only a redefinition of the parameter α2 )
we neglect cell death, we will also keep n = 1 for the reason mentioned before. From the
preceeding paragraph we see that tha population of g cannot grow for α1 = α2 , that is
when the time for cell division (α1)^(-1) is equal to the generation time of somatic cells (α2)^(-1) .
Both cell types have the same growth rate, in our system it means that for each germinal
cell division there is one recombination of the CRE-LOX box and the generation of one
somatic cell. This means that we need less than 50% recombination probability in order
for our system to work. For recombination rates α2 (for fixed α1 ) close to this limit, the
growth rate over population size curve as a function α2 is a straight line with negative
slope g'/g = α1 − α2 , going to zero at 50% recombination probability.