Caltech/Project
From 2007.igem.org
| (2 intermediate revisions not shown) | |||
| Line 15: | Line 15: | ||
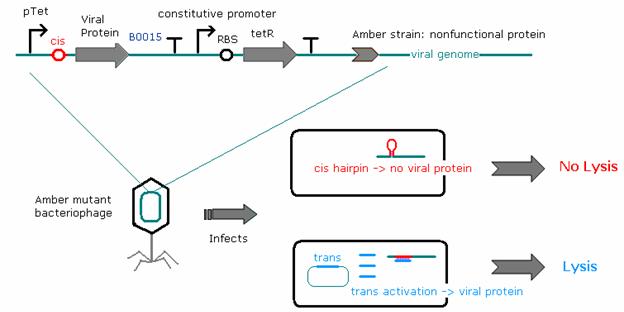
As an initial mechanism to target viruses to specific cell types, we will place the viral developmental genes under riboregulator control. Viral mRNAs for the regulated developmental genes will express with a stem loop sequestering ribosome binding sites, preventing translation. Specific mRNA in target E. coli will invade the stem loop, freeing the ribosome binding site and allowing proper translation. We believe this approach is more general than methods which might target specific cell-surface markers. Furthermore, if this method works, it would be possible in principle to extend viral mRNA regulation using aptamers capable of recognizing subtle signals such as post-translational modification. | As an initial mechanism to target viruses to specific cell types, we will place the viral developmental genes under riboregulator control. Viral mRNAs for the regulated developmental genes will express with a stem loop sequestering ribosome binding sites, preventing translation. Specific mRNA in target E. coli will invade the stem loop, freeing the ribosome binding site and allowing proper translation. We believe this approach is more general than methods which might target specific cell-surface markers. Furthermore, if this method works, it would be possible in principle to extend viral mRNA regulation using aptamers capable of recognizing subtle signals such as post-translational modification. | ||
| - | We selected the viral developmental genes <i>N, Q,</i> and <i>cro</i> as promising targets for regulation. <i>N</i> and <i>Q</i> are antiterminators required for λ to transcribe its full set of genes. Viruses lacking these genes stall at extremely early developmental stages and are completely inviable, barely producing any viral mRNA. <i>cro</i> biases | + | We selected the viral developmental genes <i>N, Q,</i> and <i>cro</i> as promising targets for regulation. <i>N</i> and <i>Q</i> are antiterminators required for λ to transcribe its full set of genes. Viruses lacking these genes stall at extremely early developmental stages and are completely inviable, barely producing any viral mRNA. <i>cro</i> biases the virus' decision to either lyse a target cell or integrate into the host's DNA, possibly allowing us to selectively 'rewire' host bacteria. |
| - | lyse a target cell or integrate into | + | |
Choosing an appropriate λ strain poses a challenge. Existing strains with defective <i>N, Q,</i> and <i>cro</i> genes lack unique restriction sites to clone our constructs into. Therefore, we will utilize recombineering to introduce introduce these mutations into phages specifically designed to accept cloning inserts. | Choosing an appropriate λ strain poses a challenge. Existing strains with defective <i>N, Q,</i> and <i>cro</i> genes lack unique restriction sites to clone our constructs into. Therefore, we will utilize recombineering to introduce introduce these mutations into phages specifically designed to accept cloning inserts. | ||
| Line 50: | Line 49: | ||
We see several applications for this research. Most immediately, we believe that selective bacteriophage could be used for containment in the event of an accidental environmental release of engineered bacteria. The phage could specifically target the released bacteria, without harming other wild species of bacteria. | We see several applications for this research. Most immediately, we believe that selective bacteriophage could be used for containment in the event of an accidental environmental release of engineered bacteria. The phage could specifically target the released bacteria, without harming other wild species of bacteria. | ||
| - | Next, the phage could be used in a laboratory setting to help prevent the formation of 'cheaters' in an engineered population. If a genetic construct places host cells at a growth disadvantage, and cells which can eliminate the construct would quickly take over the population. Our phage could be targeted such that | + | Next, the phage could be used in a laboratory setting to help prevent the formation of 'cheaters' in an engineered population. If a genetic construct places host cells at a growth disadvantage, and cells which can eliminate the construct would quickly take over the population. Our phage could be targeted such that they only kill cells which no longer display their desired function, allowing for more direct control over cheating. |
Finally, the lessons learned using our system may be applicable to medical applications involving gene therapy. In such an application, we would aim to target specific cells. These could allow tissue-specific treatment, or specific targeting of cancerous cells. | Finally, the lessons learned using our system may be applicable to medical applications involving gene therapy. In such an application, we would aim to target specific cells. These could allow tissue-specific treatment, or specific targeting of cancerous cells. | ||
|} | |} | ||
</div> | </div> | ||
Latest revision as of 03:54, 27 October 2007
iGEM 2007
Home
Highlights
Project
People
Protocols
|