Tokyo/IPTG assay
From 2007.igem.org
m (→Samples:) |
(→Procedure:) |
||
| (2 intermediate revisions not shown) | |||
| Line 16: | Line 16: | ||
<br>A4 ΔP + Lux-lac hybrid promter ([http://partsregistry.org/Part:BBa_I751101 BBa_I751101]) into pBR322 cell (LacI non-producing cell) | <br>A4 ΔP + Lux-lac hybrid promter ([http://partsregistry.org/Part:BBa_I751101 BBa_I751101]) into pBR322 cell (LacI non-producing cell) | ||
<br>A4 ΔP + Lux-lac hybrid promter ([http://partsregistry.org/Part:BBa_I751101 BBa_I751101]) into pTrc99A cell (LacI producing cell) | <br>A4 ΔP + Lux-lac hybrid promter ([http://partsregistry.org/Part:BBa_I751101 BBa_I751101]) into pTrc99A cell (LacI producing cell) | ||
| + | |||
| + | A LacI-source plasmid, pTrc99A, can be substituted with other pBR-derivatives, such as pET plasmids. | ||
===Procedure: === | ===Procedure: === | ||
| Line 21: | Line 23: | ||
<br>prepare overnight culture for each sample | <br>prepare overnight culture for each sample | ||
<br>make fresh culture | <br>make fresh culture | ||
| - | <br>take 3 ul of the | + | <br>take 3 ul of the overnight culture into 3 ml of LB (Amp and/or Kan) in Falcon tubes. |
<br>incubate for 2 to 3 hours until the observed OD is around 0.5 | <br>incubate for 2 to 3 hours until the observed OD is around 0.5 | ||
<br>add AHL & IPTG solution | <br>add AHL & IPTG solution | ||
| Line 27: | Line 29: | ||
<br>[IPTG]final (in 3 ml LB culture) = 1000, 100, 10, 1, 0.1, 0.01, and 0 mM | <br>[IPTG]final (in 3 ml LB culture) = 1000, 100, 10, 1, 0.1, 0.01, and 0 mM | ||
<br>incubate for 2 to 3 hours | <br>incubate for 2 to 3 hours | ||
| - | <br>apply 150 ul of samples into 96-well | + | <br>apply 150 ul of samples into 96-well plate |
<br>FLA measurement | <br>FLA measurement | ||
Latest revision as of 05:09, 27 October 2007
Works top 0.Hybrid promoter 1.Formulation 2.Assay1 3.Simulation 4.Assay2 5.Future works
Objective of this assay Effect of AHL Effect of LacI-IPTG Preliminary assays
LacI-IPTG assay
Objective:
By testing how LacI represses lux-lac hybrid promoter, parameters(n3,K3) should be decided. In order to adjust effective concentration of LacI, IPTG was added.
The order of the concentration is used for more detailed assay with narrower range of the IPTG concentration.
Samples:
in DH5 alpha
A4 pcI([http://partsregistry.org/Part:BBa_I751103 BBa_I751103]) into pTrc99A cell (LacI producing cell)
A4 ΔP([http://partsregistry.org/Part:BBa_I751100 BBa_I751100]) into pTrc99A cell (LacI producing cell)
A4 ΔP + Lux-lac hybrid promter ([http://partsregistry.org/Part:BBa_I751101 BBa_I751101]) into pBR322 cell (LacI non-producing cell)
A4 ΔP + Lux-lac hybrid promter ([http://partsregistry.org/Part:BBa_I751101 BBa_I751101]) into pTrc99A cell (LacI producing cell)
A LacI-source plasmid, pTrc99A, can be substituted with other pBR-derivatives, such as pET plasmids.
Procedure:
prepare overnight culture for each sample
make fresh culture
take 3 ul of the overnight culture into 3 ml of LB (Amp and/or Kan) in Falcon tubes.
incubate for 2 to 3 hours until the observed OD is around 0.5
add AHL & IPTG solution
[AHL]final (in 3 ml LB culture) = 10 nM
[IPTG]final (in 3 ml LB culture) = 1000, 100, 10, 1, 0.1, 0.01, and 0 mM
incubate for 2 to 3 hours
apply 150 ul of samples into 96-well plate
FLA measurement
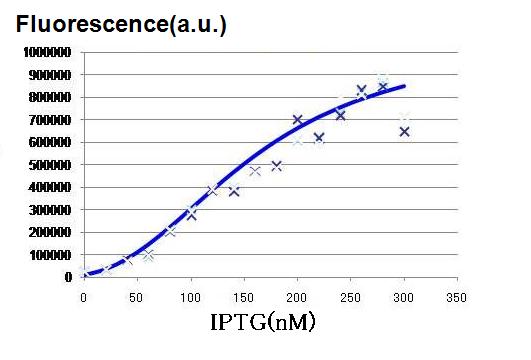
Result & Conclusion:
As the concentration of IPTG increases, GFP fluorescence increased, indicating that the hybrid promoter in the LacI expressing cell became increasingly strengthened by decreasing repression by LacI. The activation graph in Fig. 2, the characteristics of the hybrid promoter expressed in Hill function is determined.
n3 = 2.47 (-)
K3 = 0.295 (μM)