Paris/July 12
From 2007.igem.org
Nicolas C. (Talk | contribs) |
Nicolas C. (Talk | contribs) |
||
| (23 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | [[Paris/July 11|yesterday]] -- [[Paris/July 13|tomorrow]] <br> | |
| - | + | ||
==Preparation of a stock of phage on w121:== | ==Preparation of a stock of phage on w121:== | ||
| - | + | See [[Paris/PROTOCOLS#Preparation_of_the_P1_stock_on_the_w121_strain.|Protocols]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | |||
==Titration of bacteriophages P1 == | ==Titration of bacteriophages P1 == | ||
| + | [[User:Nicolas C.|Nicolas C.]]<br> | ||
We measure the strength of former P1 preparation : | We measure the strength of former P1 preparation : | ||
* The stock prepared the 3.7.7 | * The stock prepared the 3.7.7 | ||
* The stock prepared the 8.7.7 | * The stock prepared the 8.7.7 | ||
| - | See protocols for details. | + | * The stock prepared today (12.7.7) |
| + | See [[Paris/July_13|results]].<br> | ||
| + | See [[Paris/PROTOCOLS#Titration of bacteriophages|protocols]] for details. | ||
| + | |||
| + | == Culture of FtsZ TS == | ||
| + | [[User:david.bikard|David B]]<br> | ||
| + | We want to isolate a good clone of the strain FstZ TS <br> | ||
| + | From the plate of FtsZ TS84 121: <br> | ||
| + | isolation of 6 clones of the 121.1 on LBA+tet incubated at 42°C + Culture ON at 30°C <br> | ||
| + | If one clone do not grow at 42, we'll use it to do the transduction. <br> | ||
| + | See [[Paris/July_13#Test of Ftsz TS strain| result.]] | ||
| + | |||
| + | == Acinetobacter microscopy == | ||
| + | |||
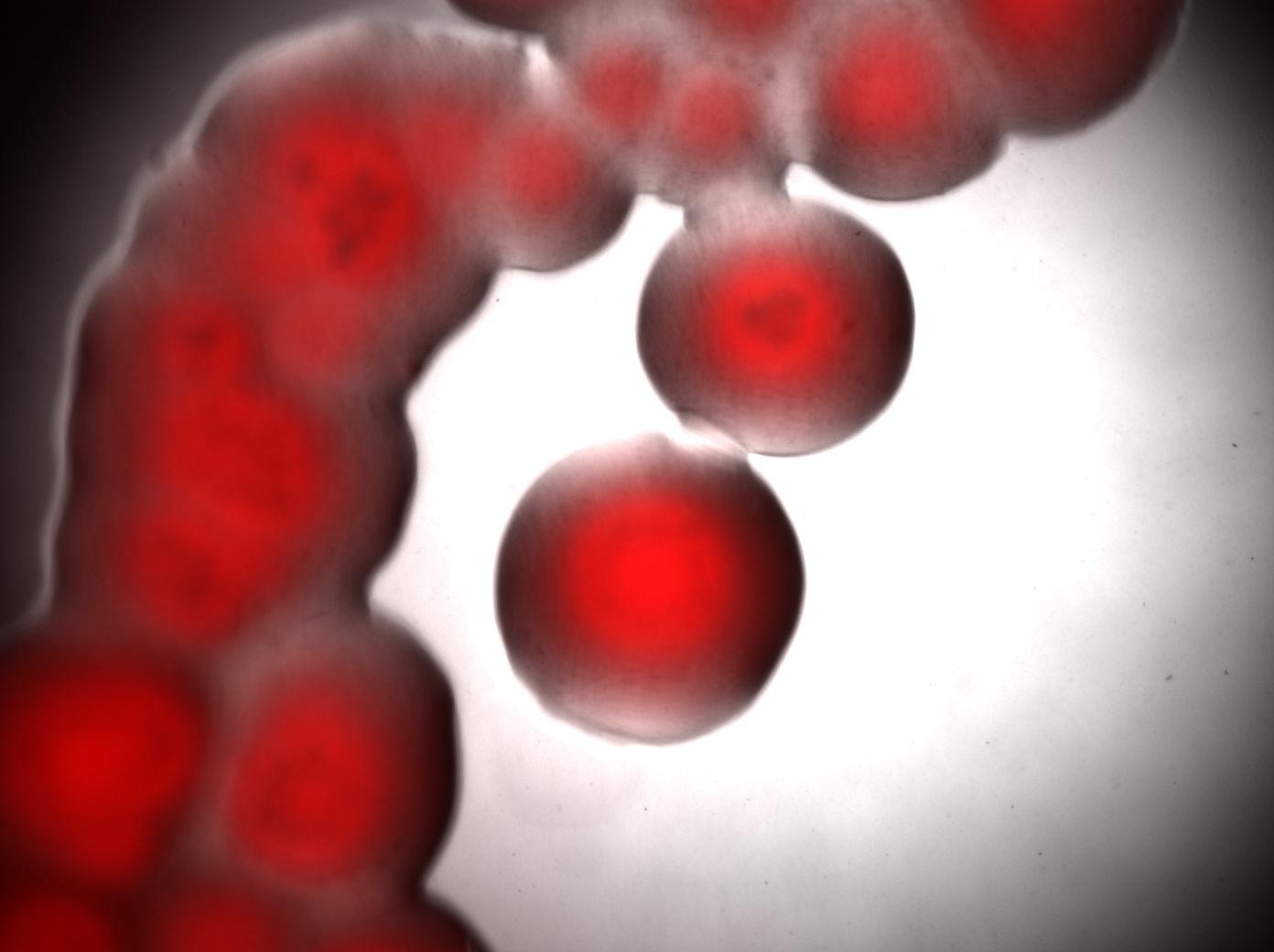
| + | We looked under 5X fluorescence microscopy the fluorescence of colonies of Acinetobacter incubated on LNMM Nile Red for 4-5 days: | ||
| + | Visualisation of colonies stained by Nile Red. It seems that only cells in the middle of colonies (i.e. "old" cells) are producing triglycerides whereas cells on the edges doesn't. Without Nile red, the colonies show no fluorescence. | ||
| + | <br> | ||
| + | [[Image:Paris_Acineto_Colonies.jpg|500px|center]] | ||
| + | |||
| + | == E.coli pKS::DGAT on LB oleate medium == | ||
| + | |||
| + | We incubated E.coli pKS::DGAT overnight on different LB medium (see [[Paris/July_11|July 11]]). | ||
| + | |||
| + | We did not observe any colony. Probably because spread plates with liquid culture kept in the freezer for one week was not a good idea... | ||
| + | |||
| + | On minimum medium, it did not grow too because what we thought to be M9 medium was only agar... | ||
Latest revision as of 17:45, 7 October 2007
Contents |
Preparation of a stock of phage on w121:
See Protocols
Titration of bacteriophages P1
Nicolas C.
We measure the strength of former P1 preparation :
- The stock prepared the 3.7.7
- The stock prepared the 8.7.7
- The stock prepared today (12.7.7)
See results.
See protocols for details.
Culture of FtsZ TS
David B
We want to isolate a good clone of the strain FstZ TS
From the plate of FtsZ TS84 121:
isolation of 6 clones of the 121.1 on LBA+tet incubated at 42°C + Culture ON at 30°C
If one clone do not grow at 42, we'll use it to do the transduction.
See result.
Acinetobacter microscopy
We looked under 5X fluorescence microscopy the fluorescence of colonies of Acinetobacter incubated on LNMM Nile Red for 4-5 days:
Visualisation of colonies stained by Nile Red. It seems that only cells in the middle of colonies (i.e. "old" cells) are producing triglycerides whereas cells on the edges doesn't. Without Nile red, the colonies show no fluorescence.
E.coli pKS::DGAT on LB oleate medium
We incubated E.coli pKS::DGAT overnight on different LB medium (see July 11).
We did not observe any colony. Probably because spread plates with liquid culture kept in the freezer for one week was not a good idea...
On minimum medium, it did not grow too because what we thought to be M9 medium was only agar...