Tokyo/Works/Assay2
From 2007.igem.org
(→Expression level check on two different ''promoters + plasmid sets'') |
(→Expression level check on two different ''promoters + plasmid sets'') |
||
| Line 23: | Line 23: | ||
====⇒see more [[Tokyo/Activation check by cell-produced AHL|details]]==== | ====⇒see more [[Tokyo/Activation check by cell-produced AHL|details]]==== | ||
| - | ==Expression level check on | + | ==Expression level check on ''promoters + plasmid sets'' of both A and B sides== |
[[Image:pC1GFP.jpg|thumb|300px| '''Fig.2''']] | [[Image:pC1GFP.jpg|thumb|300px| '''Fig.2''']] | ||
'''Purpose ''' | '''Purpose ''' | ||
| - | To | + | To test and compare the gene expression level of each side, A and B. |
| - | Lambda cI-regulated promoter and the lux lac hybrid promoter | + | Since the cell type - A or B - is detected based on the fluorescence, its activity should be measured and standardized beforehand. |
| + | Here we used the same fluorescent protein GFP on the both promoter + plasmid sets actually used in our model, where A side consists of Lambda cI-regulated promoter, and B side the lux lac hybrid promoter. | ||
[[Image:promoter hikaku.JPG|300px]] | [[Image:promoter hikaku.JPG|300px]] | ||
Revision as of 03:57, 25 October 2007
Works top 0.Hybrid promoter 1.Formulation 2.Assay1 3.Simulation 4.Assay2 5.Future works
Parameters for the equations in Formulation <←link> have been experimentally determined in Assay1 <←link>. Analysing the result, the following experiments were turned out to be necessary.
Activation check by cell-produced AHL
Purpose
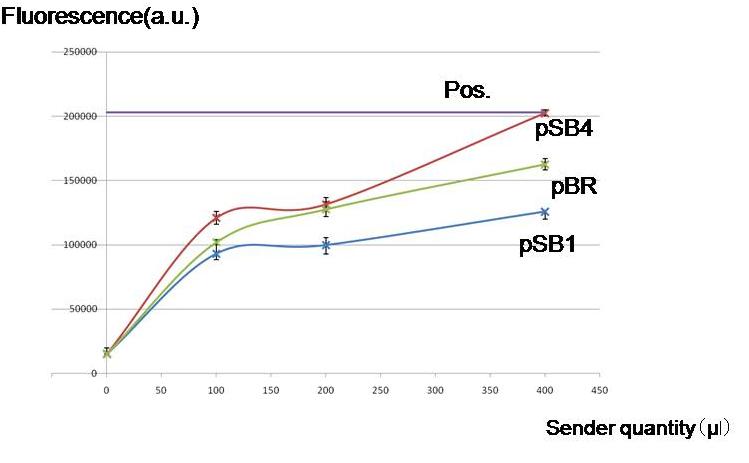
To check if worker E. coli (Sender) can produce enough AHL for our model to work by using different copy numbers of plasmids.
Result & Conclusion
Not only high copy number plasmid pSB1, also low copy number plasmid pSB4 and pBR produced enough AHL to activate the LacI hybrid promoter in other cells. Especially, pBR remarkably produced AHL in the present experiment.
⇒see more details
Expression level check on promoters + plasmid sets of both A and B sides
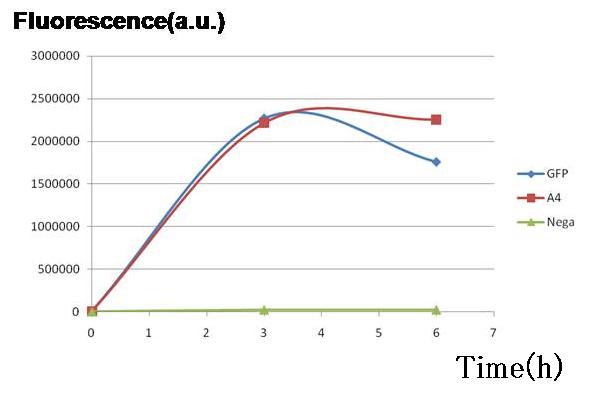
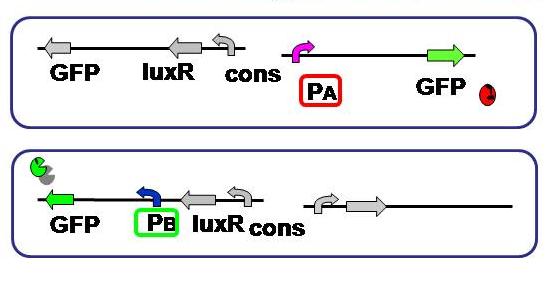
Purpose To test and compare the gene expression level of each side, A and B. Since the cell type - A or B - is detected based on the fluorescence, its activity should be measured and standardized beforehand. Here we used the same fluorescent protein GFP on the both promoter + plasmid sets actually used in our model, where A side consists of Lambda cI-regulated promoter, and B side the lux lac hybrid promoter.
Result & Conclusion
Two plasmid sets, A4Δp+pc1-GFP and A4 hybrid+GFP PBR322TetR (+)AHL, shows almost the same fluorescence of GFP, indicating that expression levels of both sets are almost the same though the latter is a bit smaller.